β-Silyl alkynoates: Versatile reagents for biocompatible and selective amide bond formation
- PMID: 39292777
- PMCID: PMC11421574
- DOI: 10.1126/sciadv.adp7544
β-Silyl alkynoates: Versatile reagents for biocompatible and selective amide bond formation
Abstract
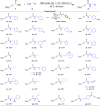
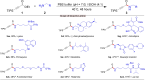
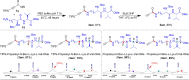
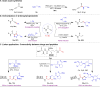
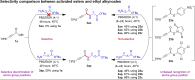
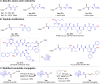
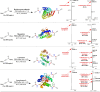
The study introduces a previously unidentified method for amide bond formation that addresses several limitations of conventional approaches. It uses the β-silyl alkynoate molecule, where the alkynyl group activates the ester for efficient amide formation, while the bulky TIPS (triisopropylsilane) group prevents unwanted 1,4-addition reactions. This approach exhibits high chemoselectivity for amines, making the method compatible with a wide range of substrates, including secondary amines, and targets the specific ε-amino group of lysine among the native amino ester's derivatives. It maintains stereochemistry during amide bond formation and TIPS group removal, allowing a versatile platform for postsynthesis modifications such as click reactions and peptide-drug conjugations. These advancements hold substantial promise for pharmaceutical development and peptide engineering, opening avenues for research applications.
Figures


References
-
- Coin I., Beyermann M., Bienert M., Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2, 3247–3256 (2007). - PubMed
-
- Keller M., Blöchl E., Wächtershäuser G., Stetter K. O., Formation of amide bonds without a condensation agent and implications for origin of life. Nature 368, 836–838 (1994). - PubMed
-
- Pattabiraman V. R., Bode J. W., Rethinking amide bond synthesis. Nature 480, 471–479 (2011). - PubMed
LinkOut - more resources
Full Text Sources

