ACSL6-activated IL-18R1-NF-κB promotes IL-18-mediated tumor immune evasion and tumor progression
- PMID: 39292786
- PMCID: PMC11409972
- DOI: 10.1126/sciadv.adp0719
ACSL6-activated IL-18R1-NF-κB promotes IL-18-mediated tumor immune evasion and tumor progression
Abstract
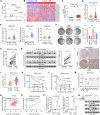
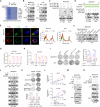
Aberrant activation of IL-18 signaling regulates tumor immune evasion and progression. However, the underlying mechanism remains unclear. Here, we report that long-chain acyl-CoA synthase 6 (ACSL6) is highly expressed in liver cancer and correlated with poor prognosis. ACSL6 promotes tumor growth, metastasis, and immune evasion mediated by IL-18, independent of its metabolic enzyme activity. Mechanistically, upon IL-18 stimulation, ACSL6 is phosphorylated by ERK2 at S674 and recruits IL-18RAP to interact with IL-18R1, thereby reinforcing the IL-18R1-IL-18RAP heterodimer and triggering NF-κB-dependent gene expression to facilitate tumor development. Furthermore, the up-regulation of CXCL1 and CXCL5 by ACSL6 promotes tumor-associated neutrophil and tumor-associated macrophage recruitment, thereby inhibiting cytotoxic CD8+ T cell infiltration. Ablation or S674A mutation of ACSL6 potentiated anti-PD-1 therapeutic efficacy by increasing the effector activity of intertumoral CD8+ T cells. We revealed that ACSL6 is a potential adaptor that activates IL-18-NF-κB axis-mediated tumor immune evasion and provides valuable insights for developing effective immunotherapy strategies for cancer.
Figures






References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
-
- Vogel A., Meyer T., Sapisochin G., Salem R., Saborowski A., Hepatocellular carcinoma. Lancet 400, 1345–1362 (2022). - PubMed
-
- Finn R. S., Qin S. K., Ikeda M., Galle P. R., Ducreux M., Kim T. Y., Kudo M., Breder V., Merle P., Kaseb A. O., Li D. N., Verret W., Xu D. Z., Hernandez S., Liu J., Huang C., Mulla S., Wang Y. L., Lim H. Y., Zhu A. X., Cheng A. L., Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020). - PubMed
-
- Gordon S. R., Aute R. L. M., Dulken B. W., Hutter G., George B. M., Ccracken M. N. M., Gupta R., Tsai J. M., Sinha R., Corey D., Ring A. M., Connolly A. J., Weissman I. L., PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

