CCR2 cooperativity promotes hematopoietic stem cell homing to the bone marrow
- PMID: 39292787
- PMCID: PMC11409967
- DOI: 10.1126/sciadv.adq1476
CCR2 cooperativity promotes hematopoietic stem cell homing to the bone marrow
Abstract
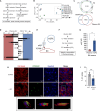
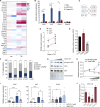
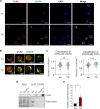
Cross-talk between hematopoietic stem and progenitor cells (HSPCs) and bone marrow (BM) cells is critical for homing and sustained engraftment after transplantation. In particular, molecular and physical adaptation of sinusoidal endothelial cells (ECs) promote HSPC BM occupancy; however, signals that govern these events are not well understood. Extracellular vesicles (EVs) are mediators of cell-cell communication crucial in shaping tissue microenvironments. Here, we demonstrate that integrin α4β7 on murine HSPC EVs targets uptake into ECs. In BM ECs, HSPC EVs induce up-regulation of C-C motif chemokine receptor 2 (CCR2) ligands that synergize with CXCL12-CXCR4 signaling to promote BM homing. In nonirradiated murine models, marrow preconditioning with HSPC EVs or recombinant CCR2 ligands improves homing and early graft occupancy after transplantation. These findings identify a role for HSPC EVs in remodeling ECs, newly define CCR2-dependent graft homing, and inform novel translational conditioning strategies to improve HSPC transplantation.
Figures




References
-
- Nombela-Arrieta C., Pivarnik G., Winkel B., Canty K. J., Harley B., Mahoney J. E., Park S.-Y., Lu J., Protopopov A., Silberstein L. E., Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat. Cell Biol. 15, 533–543 (2013). - PMC - PubMed
-
- Wright D. E., Wagers A. J., Gulati A. P., Johnson F. L., Weissman I. L., Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001). - PubMed
-
- Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E. A., Huff I. V., Junt T., Wagers A. J., Mazo I. B., von Andrian U. H., Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008 (2007). - PMC - PubMed
-
- Katayama Y., Battista M., Kao W.-M., Hidalgo A., Peired A. J., Thomas S. A., Frenette P. S., Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

