Loss of intermicrovillar adhesion factor CDHR2 impairs basolateral junctional complexes in transporting epithelia
- PMID: 39292922
- PMCID: PMC11617098
- DOI: 10.1091/mbc.E24-03-0113
Loss of intermicrovillar adhesion factor CDHR2 impairs basolateral junctional complexes in transporting epithelia
Abstract
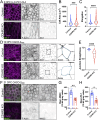
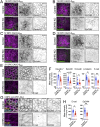
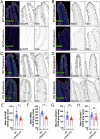
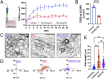
Transporting epithelial cells in the gut and kidney rely on protocadherin-based apical adhesion complexes to organize microvilli that extend into luminal space. In these systems, CDHR2 and CDHR5 localize to the distal ends of microvilli, where they form an intermicrovillar adhesion complex (IMAC) that links the tips of these structures, promotes the formation of a well-ordered array of protrusions, and thus maximizes apical membrane surface area. Recently, we discovered that IMACs can also form between microvilli that extend from neighboring cells, across cell-cell junctions. As an additional point of physical contact between cells, transjunctional IMACs are well positioned to impact the integrity of canonical tight and adherens junctions that form more basolaterally. To begin to test this idea, we examined cell culture and mouse models that lacked CDHR2 expression and were unable to form IMACs. CDHR2 knockout perturbed cell and junction morphology, reduced key components from tight and adherens junctions, impaired barrier function, and increased the motility of single cells within established monolayers. These results support the hypothesis that, in addition to organizing apical microvilli, IMACs provide a layer of cell-cell contact that functions in parallel with canonical tight and adherens junctions to promote epithelial functions.
Conflict of interest statement
Conflicts of interest: The authors declare no financial conflict of interest.
Figures

Update of
-
Loss of intermicrovillar adhesion impairs basolateral junctional complexes in transporting epithelia.bioRxiv [Preprint]. 2024 Mar 19:2024.03.19.585733. doi: 10.1101/2024.03.19.585733. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Nov 1;35(11):br21. doi: 10.1091/mbc.E24-03-0113. PMID: 38562895 Free PMC article. Updated. Preprint.
References
-
- Aman A, Piotrowski T (2008). Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell 15, 749–761. - PubMed
-
- Buckley CE, St Johnston D (2022). Apical-basal polarity and the control of epithelial form and function. Nat Rev Mol Cell Biol 23, 559–577. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

