Biallelic EPB41L3 variants underlie a developmental disorder with seizures and myelination defects
- PMID: 39292993
- PMCID: PMC11733690
- DOI: 10.1093/brain/awae299
Biallelic EPB41L3 variants underlie a developmental disorder with seizures and myelination defects
Abstract
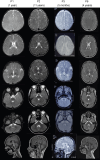
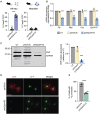
Erythrocyte membrane protein band 4.1 like 3 (EPB41L3: NM_012307.5), also known as DAL1, encodes the ubiquitously expressed, neuronally enriched 4.1B protein, part of the 4.1 superfamily of membrane-cytoskeleton adaptors. The 4.1B protein plays key roles in cell spreading, migration and cytoskeletal scaffolding that support oligodendrocyte axon adhesions essential for proper myelination. We herein describe six individuals from five unrelated families with global developmental delay, intellectual disability, seizures, hypotonia, neuroregression and delayed myelination. Exome sequencing identified biallelic variants in EPB41L3 in all affected individuals: two nonsense [c.466C>T, p.(R156*); c.2776C>T, p.(R926*)] and three frameshift [c.666delT, p.(F222Lfs*46); c.2289dupC, p.(V764Rfs*19); c.948_949delTG, p.(A317Kfs*33)]. Quantitative-real time PCR and western blot analyses of human fibroblasts harbouring EPB41L3:c.666delT, p.(F222Lfs*46) indicated ablation of EPB41L3 mRNA and 4.1B protein expression. Inhibition of the nonsense mediated decay (NMD) pathway led to an upregulation of EPB41L3:c.666delT transcripts, supporting NMD as a pathogenic mechanism. Epb41l3-deficient mouse oligodendroglia cells showed significant reduction in mRNA expression of key myelin genes, reduced branching and increased apoptosis. Our report provides the first clinical description of an autosomal recessive disorder associated with variants in EPB41L3, which we refer to as EPB41L3-associated developmental disorder (EADD). Moreover, our functional studies substantiate the pathogenicity of EPB41L3 hypothesized loss-of-function variants.
Keywords: 4.1B; delayed myelination; loss-of-function; neurodevelopmental disorder; oligodendroglia.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Q.S.P. receives a professional services fee from Merck as part of editorial duties for the Merck manual. All other authors report no competing interests.
Figures

References
-
- Baines AJ, Lu HC, Bennett PM. The protein 4.1 family: Hub proteins in animals for organizing membrane proteins. Biochim Biophys Acta. 2014;1838:605–619. - PubMed
-
- Peters LL, Weier H-UG, Walensky LD, et al. Four paralogous protein 4.1 genes map to distinct chromosomes in mouse and human. Genomics. 1998;54:348–350. - PubMed
-
- Sun C-X, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: Getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. - PubMed
-
- Parra M, Gee S, Chan N, et al. Differential domain evolution and complex RNA processing in a family of paralogous EPB41 (protein 4.1) genes facilitate expression of diverse tissue-specific isoforms. Genomics. 2004;84:637–646. - PubMed

