Increased CCL2/CCR2 axis promotes tumor progression by increasing M2 macrophages in MYC/BCL2 double-expressor DLBCL
- PMID: 39293078
- PMCID: PMC11605354
- DOI: 10.1182/bloodadvances.2024013699
Increased CCL2/CCR2 axis promotes tumor progression by increasing M2 macrophages in MYC/BCL2 double-expressor DLBCL
Abstract
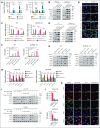
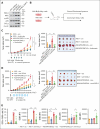
The pathogenesis of myelocytomatosis oncogene (MYC) and B-cell lymphoma 2 (BCL2) double-expressor diffuse large B-cell lymphoma (DE-DLBCL) remains unclear. To investigate how MYC and BCL2 contribute to tumor aggressiveness, we analyzed tumors from 14 patients each with DE-DLBCL and non-DE-DLBCL using whole transcriptome sequencing. Validation was performed using publicly available data sets, tumor tissues from 126 patients, DLBCL cell lines, and a syngeneic mouse lymphoma model. Our transcriptome analysis revealed significantly elevated messenger RNA levels of C-C motif chemokine ligand 2 (CCL2) and C-C chemokine receptor type 2 (CCR2) in DE-DLBCLs when compared with non-DE-DLBCLs (adjusted P value < .05). Transcriptomic analysis of public data sets and immunohistochemistry corroborated these findings, indicating increased levels of M2 macrophages but a reduction in T-cell infiltration in DE-DLBCLs when compared with non-DE-DLBCLs (all P < .05). CCR2 expression was observed mainly in tumor-infiltrating macrophages and not in DLBCL cells. Increased expression of CCL2 and CCR2 was significantly associated with a poor prognosis in patients with DLBCL. In the in vitro analyses, MYChigh/BCL2high DLBCL cells showed higher CCL2 expression and secretion than MYClow/BCL2low cells. MYC and BCL2 increased CCL2 expression and secretion by upregulation of nuclear factor κB p65 in DLBCL cells, and CCL2 promoted M2 polarization of macrophages. In a mouse lymphoma model, CCL2 contributed to the immunosuppressive microenvironment and tumor growth of MYChigh/BCL2high tumors. We demonstrated that the increased CCL2/CCR2 axis confers aggressiveness to DE-DLBCL by increasing M2 polarization and can be a potential therapeutic target.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures







References
-
- Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. International Agency for Research on Cancer; 2017. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.
-
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8(12):976–990. - PubMed
-
- Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood. 2013;122(24):3884–3891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

