UM171 enhances fitness and engraftment of gene-modified hematopoietic stem cells from patients with sickle cell disease
- PMID: 39293082
- PMCID: PMC11612367
- DOI: 10.1182/bloodadvances.2024013932
UM171 enhances fitness and engraftment of gene-modified hematopoietic stem cells from patients with sickle cell disease
Abstract
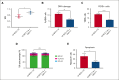
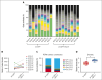
Hematopoietic stem cell (HSC) transplantation with lentiviral vector (LVV)-transduced autologous cells has proven an effective therapeutic strategy for sickle cell disease (SCD). However, ex vivo culture or proliferative stress associated with in vivo reconstitution may amplify any underlying genetic risk of leukemia. We aimed to minimize culture-induced stress and reduce genomic damage during ex vivo culture and enhance stem cell fitness and reconstitution of SCD CD34+ cells transduced with BCL11A shmiR-encoding LVV. UM171, a pyrimidoindole derivative, can expand normal HSCs during in vitro culture and has been shown to be safe and effective using umbilical cord blood. We examined the effect of UM171 during ex vivo LVV transduction of SCD HSCs. Culture of SCD CD34+ HSCs with UM171 during transduction reduced DNA damage and reactive oxygen species, decreased apoptosis, and was associated with increased numbers of immunophenotypically defined long-term HSCs. UM171 increased the engraftment of LVV-transduced human HSCs in immunodeficient mice and barcode tracing revealed increased clonal diversity of engrafting cells. In competitive transplantation assays, analysis of bone marrow showed that cells transduced in the presence of UM171 consistently outcompeted those transduced under control conditions. In summary, exposure of SCD peripheral blood CD34+ cells to UM171 during LVV transduction enhances stem cell fitness. These findings suggest manufacturing of genetically modified HSCs in the presence of UM171 may improve efficacy, safety, and sustainability of gene therapy using ex vivo approaches. BCL11A shmiR-encoding LVV is in clinical trials to treat SCD (NCT03282656), UM171 is in clinical trials to culture umbilical cord blood (NCT02668315).
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4 - PubMed
-
- Kanter J, Walters MC, Krishnamurti L, et al. Biologic and clinical efficacy of LentiGlobin for sickle cell disease. N Engl J Med. 2022;386(7):617–628. - PubMed
-
- Frangoul H, Locatelli F, Sharma A, et al. Exagamglogene autotemcel for severe sickle cell disease. N Engl J Med. 2024;390(18):1649–1662. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

