Glycan-specific IgM is critical for human immunity to Staphylococcus aureus
- PMID: 39293400
- PMCID: PMC11525025
- DOI: 10.1016/j.xcrm.2024.101734
Glycan-specific IgM is critical for human immunity to Staphylococcus aureus
Abstract
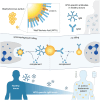
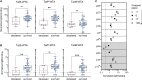
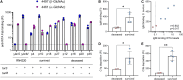
Staphylococcus aureus is a major human pathogen, yet the immune factors that protect against infection remain elusive. High titers of opsonic IgG antibodies, achieved in preclinical animal immunization studies, have consistently failed to provide protection in humans. Here, we investigate antibody responses to the conserved S. aureus surface glycan wall teichoic acid (WTA) and detect the presence of WTA-specific IgM and IgG antibodies in the plasma of healthy individuals. Functionally, WTA-specific IgM outperforms IgG in opsonophagocytic killing of S. aureus and protects against disseminated S. aureus bacteremia through passive immunization. In a clinical setting, patients with S. aureus bacteremia have significantly lower WTA-specific IgM but similar IgG levels compared to healthy controls. Importantly, low WTA-IgM levels correlate with disease mortality and impaired bacterial opsonization. Our findings may guide risk stratification of hospitalized patients and inform future design of antibody-based therapies and vaccines against serious S. aureus infection.
Keywords: IgM; Staphylococcus aureus; WTA; antibody; bacteremia; glycan; opsonic; protective immunity; protein A; wall teichoic acid.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

