A lymphocyte chemoaffinity axis for lung, non-intestinal mucosae and CNS
- PMID: 39293486
- PMCID: PMC11887596
- DOI: 10.1038/s41586-024-08043-2
A lymphocyte chemoaffinity axis for lung, non-intestinal mucosae and CNS
Abstract
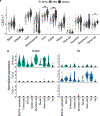
Tissue-selective chemoattractants direct lymphocytes to epithelial surfaces to establish local immune environments, regulate immune responses to food antigens and commensal organisms, and protect from pathogens. Homeostatic chemoattractants for small intestines, colon and skin are known1,2, but chemotropic mechanisms selective for respiratory tract and other non-intestinal mucosal tissues remain poorly understood. Here we leveraged diverse omics datasets to identify GPR25 as a lymphocyte receptor for CXCL17, a chemoattractant cytokine whose expression by epithelial cells of airways, upper gastrointestinal and squamous mucosae unifies the non-intestinal mucosal tissues and distinguishes them from intestinal mucosae. Single-cell transcriptomic analyses show that GPR25 is induced on innate lymphocytes before emigration to the periphery, and is imprinted in secondary lymphoid tissues on activated B and T cells responding to immune challenge. GPR25 characterizes B and T tissue resident memory cells and regulatory T lymphocytes in non-intestinal mucosal tissues and lungs in humans and mediates lymphocyte homing to barrier epithelia of the airways, oral cavity, stomach, and biliary and genitourinary tracts in mouse models. GPR25 is also expressed by T cells in cerebrospinal fluid and CXCL17 by neurons, suggesting a role in central nervous system (CNS) immune regulation. We reveal widespread imprinting of GPR25 on regulatory T cells, suggesting a mechanistic link to population genetics evidence that GPR25 is protective in autoimmunity3,4. Our results define a GPR25-CXCL17 chemoaffinity axis with the potential to integrate immunity and tolerance at non-intestinal mucosae and the CNS.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
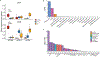
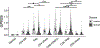

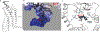






References
-
- Kunkel EJ & Butcher EC Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002). - PubMed

