Ccrk-Mak/Ick signaling is a ciliary transport regulator essential for retinal photoreceptor survival
- PMID: 39293864
- PMCID: PMC11412320
- DOI: 10.26508/lsa.202402880
Ccrk-Mak/Ick signaling is a ciliary transport regulator essential for retinal photoreceptor survival
Abstract
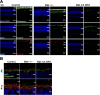
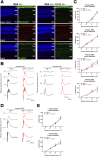
Primary cilia are microtubule-based sensory organelles whose dysfunction causes ciliopathies in humans. The formation, function, and maintenance of primary cilia depend crucially on intraflagellar transport (IFT); however, the regulatory mechanisms of IFT at ciliary tips are poorly understood. Here, we identified that the ciliopathy kinase Mak is a ciliary tip-localized IFT regulator that cooperatively acts with the ciliopathy kinase Ick, an IFT regulator. Simultaneous disruption of Mak and Ick resulted in loss of photoreceptor ciliary axonemes and severe retinal degeneration. Gene delivery of Ick and pharmacological inhibition of FGF receptors, Ick negative regulators, ameliorated retinal degeneration in Mak -/- mice. We also identified that Ccrk kinase is an upstream activator of Mak and Ick in retinal photoreceptor cells. Furthermore, the overexpression of Mak, Ick, and Ccrk and pharmacological inhibition of FGF receptors suppressed ciliopathy-related phenotypes caused by cytoplasmic dynein inhibition in cultured cells. Collectively, our results show that the Ccrk-Mak/Ick axis is an IFT regulator essential for retinal photoreceptor maintenance and present activation of Ick as a potential therapeutic approach for retinitis pigmentosa caused by MAK mutations.
© 2024 Chaya et al.
Conflict of interest statement
T Chaya and T Furukawa are inventors on a patent application related to this work filed by the Osaka University.
Figures












References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
