β2 integrins impose a mechanical checkpoint on macrophage phagocytosis
- PMID: 39294148
- PMCID: PMC11411054
- DOI: 10.1038/s41467-024-52453-9
β2 integrins impose a mechanical checkpoint on macrophage phagocytosis
Abstract
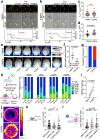
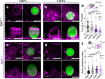
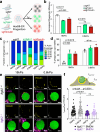
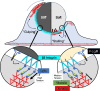
Phagocytosis is an intensely physical process that depends on the mechanical properties of both the phagocytic cell and its chosen target. Here, we employed differentially deformable hydrogel microparticles to examine the role of cargo rigidity in the regulation of phagocytosis by macrophages. Whereas stiff cargos elicited canonical phagocytic cup formation and rapid engulfment, soft cargos induced an architecturally distinct response, characterized by filamentous actin protrusions at the center of the contact site, slower cup advancement, and frequent phagocytic stalling. Using phosphoproteomics, we identified β2 integrins as critical mediators of this mechanically regulated phagocytic switch. Macrophages lacking β2 integrins or their downstream effectors, Talin1 and Vinculin, exhibited specific defects in phagocytic cup architecture and selective suppression of stiff cargo uptake. We conclude that integrin signaling serves as a mechanical checkpoint during phagocytosis to pair cargo rigidity to the appropriate mode of engulfment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R00 CA237728/CA/NCI NIH HHS/United States
- DP2 GM146337/GM/NIGMS NIH HHS/United States
- Center for Experimental Immuno-oncology Award/Memorial Sloan-Kettering Cancer Center (MSKCC)
- Graduate Research Fellowship/National Science Foundation (NSF)
- Basic Research Innovation Award/Memorial Sloan-Kettering Cancer Center (MSKCC)
LinkOut - more resources
Full Text Sources

