BWC0977, a broad-spectrum antibacterial clinical candidate to treat multidrug resistant infections
- PMID: 39294149
- PMCID: PMC11410943
- DOI: 10.1038/s41467-024-52557-2
BWC0977, a broad-spectrum antibacterial clinical candidate to treat multidrug resistant infections
Erratum in
-
Author Correction: BWC0977, a broad-spectrum antibacterial clinical candidate to treat multidrug resistant infections.Nat Commun. 2025 Jan 13;16(1):625. doi: 10.1038/s41467-025-56043-1. Nat Commun. 2025. PMID: 39805821 Free PMC article. No abstract available.
-
Author Correction: BWC0977, a broad-spectrum antibacterial clinical candidate to treat multidrug resistant infections.Nat Commun. 2025 Feb 28;16(1):2082. doi: 10.1038/s41467-025-57400-w. Nat Commun. 2025. PMID: 40021680 Free PMC article. No abstract available.
Abstract
The global crisis of antimicrobial resistance (AMR) necessitates the development of broad-spectrum antibacterial drugs effective against multi-drug resistant (MDR) pathogens. BWC0977, a Novel Bacterial Topoisomerase Inhibitor (NBTI) selectively inhibits bacterial DNA replication via inhibition of DNA gyrase and topoisomerase IV. BWC0977 exhibited a minimum inhibitory concentration (MIC90) of 0.03-2 µg/mL against a global panel of MDR Gram-negative bacteria including Enterobacterales and non-fermenters, Gram-positive bacteria, anaerobes and biothreat pathogens. BWC0977 retains activity against isolates resistant to fluoroquinolones (FQs), carbapenems and colistin and demonstrates efficacy against multiple pathogens in two rodent species with significantly higher drug levels in the epithelial lining fluid of infected lungs. In healthy volunteers, single-ascending doses of BWC0977 administered intravenously ( https://clinicaltrials.gov/study/NCT05088421 ) was found to be safe, well tolerated (primary endpoint) and achieved dose-proportional exposures (secondary endpoint) consistent with modelled data from preclinical studies. Here, we show that BWC0977 has the potential to treat a range of critical-care infections including MDR bacterial pneumonias.
© 2024. The Author(s).
Conflict of interest statement
The authors Shahul Hameed P, Harish Kotakonda, Sreevalli Sharma, Radha Nandishaiah, Nainesh Katagihallimath, Ranga Rao, Abhijeeth Chandrasekaran, Ed Griffen, Dhanashree Pillai, Sambasiva Reddy, Nagakumar Bharatham, Suryanarayanan Venkatesan, Venugopal Jonnalagadda, Maitrayee Sharma Savitha Raveendran, Sreenath Rajagopal, Harikrishna Tumma, Santanu Datta, Vasan Sambandamurthy, Vasanthi Ramachandran, Robert Clay, John Tomayko, and V. Balasubramanian declare their competing interests as either current or former employees or consultants of Bugworks Research India Pvt. Ltd. Further, authors Shahul Hameed P., Nagakumar Bharatham, Nainesh Katagihallimath, Sreevalli Sharma, & Radha Nandishaiah are inventors on the patent WO2017199265A1, which covers compounds 1 – 4 disclosed in the manuscript; and authors Shahul Hameed P., Nagakumar Bharatham, Nainesh Katagihallimath, Sreevalli Sharma, Radha Nandishaiah, Vasanthi Ramachandran and V. Balasubramanian are inventors on the patent WO2018225097A1, which covers compounds 5– 9 disclosed in the manuscript. The authors Claire Sadler, Ian Slater, Michael Morton, Amy Watters, Holly Becker, Jill Lindley, Robert Flamm, Michael Huband, Dan Sahm, Meredith Hackel, Tarun Mathur, Ruwanthi Kolamunnage-Dona, Jennifer Unsworth, Laura Mcentee, Nikki Farrington, Dhanasekaran Manickam, Chandrashekara Narayana, Sivakandan Jayachandiran, Hrushikesava Reddy, Sathya Shanker, Ramesh Jayaraman, Mahesh Nanjundappa, Vijay Richard, Teby Thomas, Savitha Nagaraj, and Shampa Das declare no competing interests.
Figures
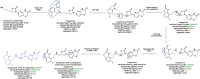

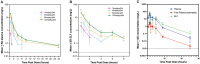
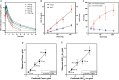
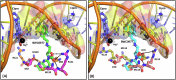

References
-
- Jim O’Neill. Nat. Rev. Drug Discov. 15, 526 (2016). - PubMed
-
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019. https://stacks.cdc.gov/view/cdc/8253210.15620/cdc:82532 (2019).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

