Germline variant affecting p53β isoforms predisposes to familial cancer
- PMID: 39294166
- PMCID: PMC11410958
- DOI: 10.1038/s41467-024-52551-8
Germline variant affecting p53β isoforms predisposes to familial cancer
Abstract
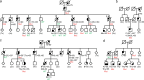
Germline and somatic TP53 variants play a crucial role during tumorigenesis. However, genetic variations that solely affect the alternatively spliced p53 isoforms, p53β and p53γ, are not fully considered in the molecular diagnosis of Li-Fraumeni syndrome and cancer. In our search for additional cancer predisposing variants, we identify a heterozygous stop-lost variant affecting the p53β isoforms (p.*342Serext*17) in four families suspected of an autosomal dominant cancer syndrome with colorectal, breast and papillary thyroid cancers. The stop-lost variant leads to the 17 amino-acid extension of the p53β isoforms, which increases oligomerization to canonical p53α and dysregulates the expression of p53's transcriptional targets. Our study reveals the capacity of p53β mutants to influence p53 signalling and contribute to the susceptibility of different cancer types. These findings underscore the significance of p53 isoforms and the necessity of comprehensive investigation into the entire TP53 gene in understanding cancer predisposition.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Wendt, C. & Margolin, S. Identifying breast cancer susceptibility genes—a review of the genetic background in familial breast cancer. Acta Oncol.58, 135–146 (2019). - PubMed
-
- Chubb, D. et al. Genetic diagnosis of high-penetrance susceptibility for colorectal cancer (CRC) is achievable for a high proportion of familial CRC by exome sequencing. J. Clin. Oncol.33, 426–432 (2015). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

