UPLC-PDA factorial design assisted method for simultaneous determination of oseltamivir, dexamethasone, and remdesivir in human plasma
- PMID: 39294224
- PMCID: PMC11411088
- DOI: 10.1038/s41598-024-71413-3
UPLC-PDA factorial design assisted method for simultaneous determination of oseltamivir, dexamethasone, and remdesivir in human plasma
Abstract
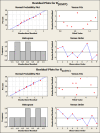
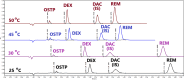
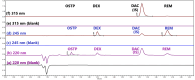
A green and simple UPLC method was developed and optimized, adopting a factorial design for simultaneous determination of oseltamivir phosphate and remdesivir with dexamethasone as a co-administered drug in human plasma and using daclatasvir dihydrochloride as an internal standard within 5 min. The separation was established on UPLC column BEH C18 1.7 μm (2.1 100.0 mm) connected to UPLC pre-column BEH 1.7 μm (2.1 5.0 mm) at 50 °C with an injection volume of 10 μL. The photodiode array detector (PDA) was set at three wavelengths of 220, 315, and 245 nm for oseltamivir phosphate, the internal standard, and both dexamethasone and remdesivir, respectively. The mobile phase consisted of methanol and ammonium acetate solution (40 mM) adjusted to pH 4 in a ratio of 61.5:38.5 (v/v) with a flow rate of 0.25 mL min-1. The calibration curves were linear over 500.0-5000.0 ng mL-1 for oseltamivir phosphate, over 10.0-500.0 ng mL-1 and 500.0-5000.0 ng mL-1 for dexamethasone, and over 20.0-500 ng mL-1 and 500.0-5000.0 ng mL-1 for remdesivir. The Gibbs free energy and Van't Hoff plots were used to investigate the effect of column oven temperatures on retention times. Fluoride-EDTA anticoagulant showed inhibition activity on the esterase enzyme in plasma. The proposed method was validated according to the M10 ICH, FDA, and EMA's bioanalytical guidelines. According to Eco-score, GAPI, and AGREE criteria, the proposed method was considered acceptable green.
Keywords: Fluoride-EDTA plasma; Gibbs free energy; Green analysis; UPLC-PDA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Development of UPLC method for simultaneous assay of some COVID-19 drugs utilizing novel instrumental standard addition and factorial design.Sci Rep. 2023 Apr 4;13(1):5466. doi: 10.1038/s41598-023-32405-x. Sci Rep. 2023. PMID: 37016018 Free PMC article.
-
Quantitative determination of oseltamivir and oseltamivir carboxylate in human fluoride EDTA plasma including the ex vivo stability using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Apr 1;891-892:57-63. doi: 10.1016/j.jchromb.2012.02.026. Epub 2012 Feb 23. J Chromatogr B Analyt Technol Biomed Life Sci. 2012. PMID: 22418071
-
Validation of LC-MS/MS methods for determination of remdesivir and its metabolites GS-441524 and GS-704277 in acidified human plasma and their application in COVID-19 related clinical studies.Anal Biochem. 2021 Mar 15;617:114118. doi: 10.1016/j.ab.2021.114118. Epub 2021 Jan 26. Anal Biochem. 2021. PMID: 33508271 Free PMC article.
-
Development and validation of a simple, selective, and sensitive LC-MS/MS assay for the quantification of remdesivir in human plasma.J Chromatogr B Analyt Technol Biomed Life Sci. 2021 May 1;1171:122641. doi: 10.1016/j.jchromb.2021.122641. Epub 2021 Mar 10. J Chromatogr B Analyt Technol Biomed Life Sci. 2021. PMID: 33756448 Free PMC article.
-
Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid-19 treated patient.Clin Chem Lab Med. 2020 Jun 22;58(9):1461-1468. doi: 10.1515/cclm-2020-0612. Print 2020 Aug 27. Clin Chem Lab Med. 2020. PMID: 32573468
References
-
- Vafaei, S., Razmi, M., Mansoori, M., Asadi-Lari, M. & Madjd, Z. Spotlight of remdesivir in comparison with ribavirin, favipiravir, oseltamivir and umifenovir in coronavirus disease 2019 (COVID-19) pandemic. Lancet Infect. Dis.10.2139/ssrn.3569866 (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

