Efficient combinatorial adaptor-mediated targeting of acute myeloid leukemia with CAR T-cells
- PMID: 39294295
- PMCID: PMC11588662
- DOI: 10.1038/s41375-024-02409-1
Efficient combinatorial adaptor-mediated targeting of acute myeloid leukemia with CAR T-cells
Abstract
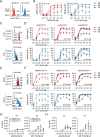
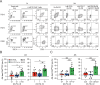
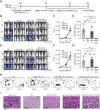
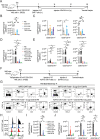
CAR T-cell products targeting lineage-specific cell-of-origin antigens, thereby eliminating both tumor and healthy counterpart cells, are currently clinically approved therapeutics in B- and plasma-cell malignancies. While they represent a major clinical improvement, they are still limited in terms of efficacy by e.g. single, sometimes low-expressed antigen targeting, and in terms of safety by e.g., lack of on-off activity. Successful cell-of-origin non-discriminative targeting of heterogeneous hematopoietic stem and progenitor cell malignancies, such as acute myeloid leukemia (AML), will require antigen-versatile targeting and off-switching of effectors in order to then allow rescue by hematopoietic stem cell transplantation (HSCT), preventing permanent myeloablation. To address this, we developed adaptor-CAR (AdFITC-CAR) T-cells targeting fluoresceinated AML antigen-binding diabody adaptors. This platform enables the use of adaptors matching the AML-antigen-expression profile and conditional activity modulation. Combining adaptors significantly improved lysis of AML cells in vitro. In therapeutic xenogeneic mouse models, AdFITC-CAR T-cells co-administered with single diabody adaptors were as efficient as direct CAR T-cells, and combinatorial use of adaptors further enhanced therapeutic efficacy against both, cell lines and primary AML. Collectively, this study provides proof-of-concept that AdFITC-CAR T-cells and combinations of adaptors can efficiently enhance immune-targeting of AML.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. - PubMed
-
- San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos M-V, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med 2023;389:335–47. - PubMed
MeSH terms
Substances
Grants and funding
- 310030_184747/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030B_182003/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- KFS-3846-02-2016/Krebsliga Schweiz (Ligue Suisse Contre le Cancer)
- Clinical Research Priority Program "ImmunoCure" of the University of Zurich/Universität Zürich (University of Zurich)
- University Research Priority Project Translational Cancer Research/Universität Zürich (University of Zurich)
LinkOut - more resources
Full Text Sources
Medical

