Dual role of the S5 segment in type 1 ryanodine receptor channel gating
- PMID: 39294299
- PMCID: PMC11411075
- DOI: 10.1038/s42003-024-06787-1
Dual role of the S5 segment in type 1 ryanodine receptor channel gating
Abstract
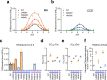
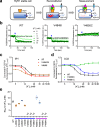
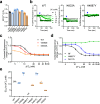
The type 1 ryanodine receptor (RyR1) is a Ca2+ release channel in the sarcoplasmic reticulum that is essential for skeletal muscle contraction. RyR1 forms a channel with six transmembrane segments, in which S5 is the fifth segment and is thought to contribute to pore formation. However, its role in channel gating remains unclear. Here, we performed a functional analysis of several disease-associated mutations in S5 and interpreted the results with respect to the published RyR1 structures to identify potential interactions associated with the mutant phenotypes. We demonstrate that S5 plays a dual role in channel gating: the cytoplasmic side interacts with S6 to reduce the channel activity, whereas the luminal side forms a rigid structural base necessary for S6 displacement in channel opening. These results deepen our understanding of the molecular mechanisms of RyR1 channel gating and provide insight into the divergent disease phenotypes caused by mutations in S5.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



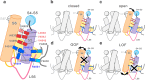
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

