Crosstalk between FTH1 and PYCR1 dysregulates proline metabolism and mediates cell growth in KRAS-mutant pancreatic cancer cells
- PMID: 39294443
- PMCID: PMC11447051
- DOI: 10.1038/s12276-024-01300-4
Crosstalk between FTH1 and PYCR1 dysregulates proline metabolism and mediates cell growth in KRAS-mutant pancreatic cancer cells
Erratum in
-
Author Correction: Crosstalk between FTH1 and PYCR1 dysregulates proline metabolism and mediates cell growth in KRAS-mutant pancreatic cancer cells.Exp Mol Med. 2025 Mar;57(3):700-701. doi: 10.1038/s12276-025-01437-w. Exp Mol Med. 2025. PMID: 40148571 Free PMC article. No abstract available.
Abstract
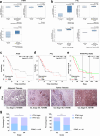
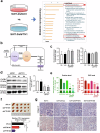
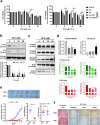
Ferritin, comprising heavy (FTH1) and light (FTL) chains, is the main iron storage protein, and pancreatic cancer patients exhibit elevated serum ferritin levels. Specifically, higher ferritin levels are correlated with poorer pancreatic ductal adenocarcinoma (PDAC) prognosis; however, the underlying mechanism and metabolic programming of ferritin involved in KRAS-mutant PDAC progression remain unclear. Here, we observed a direct correlation between FTH1 expression and cell viability and clonogenicity in KRAS-mutant PDAC cell lines as well as with in vivo tumor growth through the control of proline metabolism. Our investigation highlights the intricate relationship between FTH1 and pyrroline-5-carboxylate reductase 1 (PYCR1), a crucial mitochondrial enzyme facilitating the glutamate-to-proline conversion, underscoring its impact on proline metabolic imbalance in KRAS-mutant PDAC. This regulation is further reversed by miR-5000-3p, whose dysregulation results in the disruption of proline metabolism, thereby accentuating the progression of KRAS-mutant PDAC. Additionally, our study demonstrated that deferasirox, an oral iron chelator, significantly diminishes cell viability and tumor growth in KRAS-mutant PDAC by targeting FTH1-mediated pathways and altering the PYCR1/PRODH expression ratio. These findings underscore the novel role of FTH1 in proline metabolism and its potential as a target for PDAC therapy development.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Alkhateeb, A. A. & Connor, J. R. The significance of ferritin in cancer: anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta1836, 245–254 (2013). - PubMed
-
- Scully, R. E., Mark, E. J., McNeely, W. F., Ebeling, S. H. & Phillips, L. D. Weekly clinicopathological exercises. N. Engl. J. Med.337, 839–845 (1997). - PubMed
-
- Ji, M. et al. Clinical significance of serum ferritin in elderly patients with primary lung carcinoma. Tumour Biol.35, 10195–10199 (2014). - PubMed
-
- Facciorusso, A. et al. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J. Gastroenterol. Hepatol.29, 1905–1910 (2014). - PubMed
-
- Petekkaya, I. Impact of inflammatory markers on the prognosis of patients with operable breast cancer. J. Balk. Union Oncol.19, 673–680 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

