A cell-autonomous role for border-associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury
- PMID: 39294490
- PMCID: PMC11758676
- DOI: 10.1038/s41593-024-01757-6
A cell-autonomous role for border-associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury
Abstract
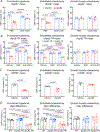
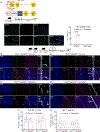
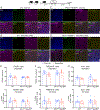
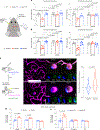
Apolipoprotein E4 (ApoE4), the strongest genetic risk factor for sporadic Alzheimer's disease, is also a risk factor for microvascular pathologies leading to cognitive impairment, particularly subcortical white matter injury. These effects have been attributed to alterations in the regulation of the brain blood supply, but the cellular source of ApoE4 and the underlying mechanisms remain unclear. In mice expressing human ApoE3 or ApoE4, we report that border-associated macrophages (BAMs), myeloid cells closely apposed to neocortical microvessels, are both sources and effectors of ApoE4 mediating the neurovascular dysfunction through reactive oxygen species. ApoE4 in BAMs is solely responsible for the increased susceptibility to oligemic white matter damage in ApoE4 mice and is sufficient to enhance damage in ApoE3 mice. The data unveil a new aspect of BAM pathobiology and highlight a previously unrecognized cell-autonomous role of BAM in the neurovascular dysfunction of ApoE4 with potential therapeutic implications.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
D.M.H. is on the scientific advisory board of C2N diagnostics and has equity. He is also on the scientific advisory board of Denali Therapeutics, Genentech and Cajal Therapeutics and consults for Asteroid. C.I. is on the scientific advisory board of Broadview Ventures. The remaining authors declare no competing interests.
Figures












Update of
-
Cell autonomous role of border associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury.Res Sq [Preprint]. 2023 Aug 4:rs.3.rs-3222611. doi: 10.21203/rs.3.rs-3222611/v1. Res Sq. 2023. Update in: Nat Neurosci. 2024 Nov;27(11):2138-2151. doi: 10.1038/s41593-024-01757-6. PMID: 37577565 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- A2022003F/BrightFocus Foundation (BrightFocus)
- K22 NS123507/NS/NINDS NIH HHS/United States
- K22-NS123507/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01-NS126467/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 NS097805/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

