Inhibiting Ca2+ channels in Alzheimer's disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia
- PMID: 39294491
- PMCID: PMC11537984
- DOI: 10.1038/s41593-024-01753-w
Inhibiting Ca2+ channels in Alzheimer's disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia
Abstract
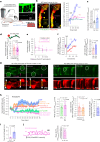
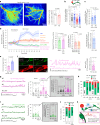
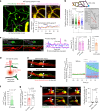
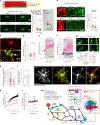
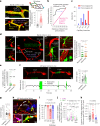
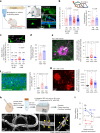
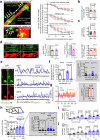
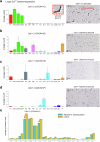
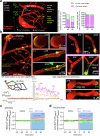
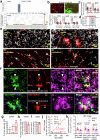
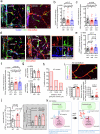
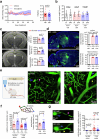
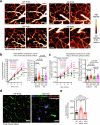
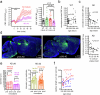
Early in Alzheimer's disease (AD), pericytes constrict capillaries, increasing their hydraulic resistance and trapping of immune cells and, thus, decreasing cerebral blood flow (CBF). Therapeutic approaches to attenuate pericyte-mediated constriction in AD are lacking. Here, using in vivo two-photon imaging with laser Doppler and speckle flowmetry and magnetic resonance imaging, we show that Ca2+ entry via L-type voltage-gated calcium channels (CaVs) controls the contractile tone of pericytes. In AD model mice, we identifed pericytes throughout the capillary bed as key drivers of an immune reactive oxygen species (ROS)-evoked and pericyte intracellular calcium concentration ([Ca2+]i)-mediated decrease in microvascular flow. Blocking CaVs with nimodipine early in disease progression improved CBF, reduced leukocyte stalling at pericyte somata and attenuated brain hypoxia. Amyloid β (Aβ)-evoked pericyte contraction in human cortical tissue was also greatly reduced by CaV block. Lowering pericyte [Ca2+]i early in AD may, thus, offer a therapeutic strategy to enhance brain energy supply and possibly cognitive function in AD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Marshall, R. S. et al. Recovery of brain function during induced cerebral hypoperfusion. Brain124, 1208–1217 (2001). - PubMed
-
- Wang, X. et al. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-β oligomerization in rats. J. Alzheimers Dis.21, 813–822 (2010). - PubMed
MeSH terms
Substances
Grants and funding
- NIH P50 HD105351/U.S. Department of Health & Human Services | NIH | Center for Scientific Review (NIH Center for Scientific Review)
- P50 HD105351/HD/NICHD NIH HHS/United States
- LiDO PhD studentship/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- 740427/EC | EC Seventh Framework Programm | FP7 Ideas: European Research Council (FP7-IDEAS-ERC - Specific Programme: "Ideas" Implementing the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007 to 2013))
- MR/X010511/1/RCUK | Medical Research Council (MRC)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

