An exploration of available methods and tools to improve the efficiency of systematic review production: a scoping review
- PMID: 39294580
- PMCID: PMC11409535
- DOI: 10.1186/s12874-024-02320-4
An exploration of available methods and tools to improve the efficiency of systematic review production: a scoping review
Abstract
Background: Systematic reviews (SRs) are time-consuming and labor-intensive to perform. With the growing number of scientific publications, the SR development process becomes even more laborious. This is problematic because timely SR evidence is essential for decision-making in evidence-based healthcare and policymaking. Numerous methods and tools that accelerate SR development have recently emerged. To date, no scoping review has been conducted to provide a comprehensive summary of methods and ready-to-use tools to improve efficiency in SR production.
Objective: To present an overview of primary studies that evaluated the use of ready-to-use applications of tools or review methods to improve efficiency in the review process.
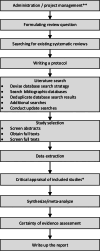
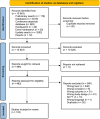
Methods: We conducted a scoping review. An information specialist performed a systematic literature search in four databases, supplemented with citation-based and grey literature searching. We included studies reporting the performance of methods and ready-to-use tools for improving efficiency when producing or updating a SR in the health field. We performed dual, independent title and abstract screening, full-text selection, and data extraction. The results were analyzed descriptively and presented narratively.
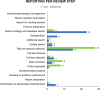
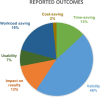
Results: We included 103 studies: 51 studies reported on methods, 54 studies on tools, and 2 studies reported on both methods and tools to make SR production more efficient. A total of 72 studies evaluated the validity (n = 69) or usability (n = 3) of one method (n = 33) or tool (n = 39), and 31 studies performed comparative analyses of different methods (n = 15) or tools (n = 16). 20 studies conducted prospective evaluations in real-time workflows. Most studies evaluated methods or tools that aimed at screening titles and abstracts (n = 42) and literature searching (n = 24), while for other steps of the SR process, only a few studies were found. Regarding the outcomes included, most studies reported on validity outcomes (n = 84), while outcomes such as impact on results (n = 23), time-saving (n = 24), usability (n = 13), and cost-saving (n = 3) were less often evaluated.
Conclusion: For title and abstract screening and literature searching, various evaluated methods and tools are available that aim at improving the efficiency of SR production. However, only few studies have addressed the influence of these methods and tools in real-world workflows. Few studies exist that evaluate methods or tools supporting the remaining tasks. Additionally, while validity outcomes are frequently reported, there is a lack of evaluation regarding other outcomes.
Keywords: Automation tools; Evidence synthesis; Method; Rapid review; Scoping review; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Large language models for conducting systematic reviews: on the rise, but not yet ready for use-a scoping review.J Clin Epidemiol. 2025 May;181:111746. doi: 10.1016/j.jclinepi.2025.111746. Epub 2025 Feb 26. J Clin Epidemiol. 2025. PMID: 40021099
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Automation of systematic reviews of biomedical literature: a scoping review of studies indexed in PubMed.Syst Rev. 2024 Jul 8;13(1):174. doi: 10.1186/s13643-024-02592-3. Syst Rev. 2024. PMID: 38978132 Free PMC article.
-
Resource use during systematic review production varies widely: a scoping review.J Clin Epidemiol. 2021 Nov;139:287-296. doi: 10.1016/j.jclinepi.2021.05.019. Epub 2021 Jun 4. J Clin Epidemiol. 2021. PMID: 34091021
-
How to conduct systematic reviews more expeditiously?Syst Rev. 2015 Nov 12;4:160. doi: 10.1186/s13643-015-0147-7. Syst Rev. 2015. PMID: 26563648 Free PMC article.
Cited by
-
A comparative study of screening performance between abstrackr and GPT models: Systematic review and contextual analysis.BMC Med Inform Decis Mak. 2025 Aug 7;25(1):293. doi: 10.1186/s12911-025-03138-w. BMC Med Inform Decis Mak. 2025. PMID: 40775694 Free PMC article.
References
-
- Oliver S, Dickson K, Bangpan M. Systematic reviews: making them policy relevant. A briefing for policy makers and systematic reviewers. London: EPPI-Centre, Social Science Research Unit, UCL Institute of Education, University College London; 2015.
-
- Donnelly CA, Boyd I, Campbell P, Craig C, Vallance P, Walport M, Whitty CJM, Woods E, Wormald C. Four principles to make evidence synthesis more useful for policy. Nature. 2018;558(7710):361–4. - PubMed
-
- Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evidence Synthesis. 2020;18(10). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials