"Comparison between high-flow nasal cannula (HFNC) therapy and noninvasive ventilation (NIV) in children with acute respiratory failure by bronchiolitis: a randomized controlled trial"
- PMID: 39294604
- PMCID: PMC11412039
- DOI: 10.1186/s12887-024-05058-6
"Comparison between high-flow nasal cannula (HFNC) therapy and noninvasive ventilation (NIV) in children with acute respiratory failure by bronchiolitis: a randomized controlled trial"
Abstract
Background: The objective of this study was to compare HFNC therapy to noninvasive ventilation (NIV/BiPAP) in children with bronchiolitis who developed respiratory failure. We hypothesized that HFNC therapy would not be inferior to NIV.
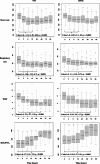
Methods: This was a noninferiority open-label randomized single-center clinical trial conducted at a tertiary Brazilian hospital. Children under 2 years of age with no chronic conditions admitted for bronchiolitis that progressed to mild to moderate respiratory distress (Wood-Downes-Férres score < 8) were randomized to either the HFNC group or NIV (BiPAP) group through sealed envelopes. Vital signs, FiO2, Wood-Downes-Férres score and HFNC/NIV parameters were recorded up to 96 h after therapy initiation. Children who developed respiratory failure despite receiving initial therapy were intubated. Crossover was not allowed. The primary outcome analyzed was invasive mechanical ventilation requirement. The secondary outcomes were sedation usage, invasive mechanical ventilation duration, the PICU LOS, the hospital LOS, and mortality rate.
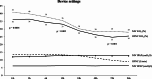
Results: A total of 126 patients were allocated to the NIV group (132 randomized and 6 excluded), and 126 were allocated to the HFNC group (136 randomized and 10 excluded). The median age was 2.5 (1-6) months in the NIV group and 3 (2-7) months in the HFNC group (p = 0,07). RSV was the most common virus isolated in both groups (72% vs. 71.4%, NIV and HFNC, respectively). Thirty-seven patients were intubated in the NIV group and 29 were intubated in the HFNC group (29% vs. 23%, p = 0.25). According to the Farrington-Manning test, with a noninferiority margin of 15%, the difference was 6.3% in favor of HFNC therapy (95% confidence interval: -4.5 to 17.1%, p < 0.0001). There was no significant difference in the PICU LOS or sedation duration. Sedation requirement, hospital LOS and invasive mechanical ventilation duration were lower in the HFNC group.
Conclusion: HFNC therapy is noninferior to NIV in infants admitted with mild to moderate respiratory distress caused by bronchiolitis that progresses to respiratory failure.
Trial registration numbers: U1111-1262-1740; RBR-104z966s. Registered 03/01/2023 (retrospectively registered). ReBEC: https://ensaiosclinicos.gov.br/rg/RBR-104z966s .
Keywords: Bronchiolitis; High-flow nasal cannula; Noninvasive ventilation; Respiratory failure.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Slain KN, Shein SL, Rotta AT. The use of high-flow nasal cannula in the pediatric emergency department. J Pediatr (Rio J). 2017. 10.1016/j.jped.2017.06.006. - PubMed
-
- Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014. 10.1097/PCC.0000000000000112. - PubMed
-
- Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015. 10.1002/ppul.23060. - PubMed
-
- Guglielmo RD, Hotz JC, Ross PA, Deakers TW, Diep JEL, Newth CJL, et al. High-flow nasal cannula reduces effort of breathing but not consistently via positive end-expiratory pressure. Chest. 2022. 10.1016/j.chest.2022.03.008. - PubMed
-
- Rotta AT, Rehder KJ. Toward elucidating the mechanism of action of high-flow nasal cannula support in children. Chest. 2022. 10.1016/j.chest.2022.04.010. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

