Enhanced anti-tumor efficacy of S3I-201 in breast cancer mouse model through Wharton jelly- exosome
- PMID: 39294673
- PMCID: PMC11409531
- DOI: 10.1186/s12935-024-03501-3
Enhanced anti-tumor efficacy of S3I-201 in breast cancer mouse model through Wharton jelly- exosome
Abstract
Objective: Exosomes, membrane-enveloped vesicles found in various cell types, including Wharton's jelly mesenchymal stem cells, play a crucial role in intercellular communication and regulation. Their use as a cell-free nanotechnology and drug delivery system has attracted attention. Triple-negative breast cancer (TNBC) is a major global health problem and is characterized by a high mortality rate. This study investigates the potential of Wharton's Jelly mesenchymal stem cell-derived exosomes (WJ-Exo) as carriers of S3I-201 and their effects on STAT3 expression in breast cancer cell lines, and evaluates whether these exosomes can enhance the anti-tumor effect of S3I-201.
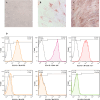
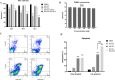
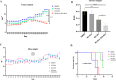
Methods: The filtered WJ-Exos were analyzed by Transmission Electron Microscopy (TEM), Scanning electron microscopy (SEM), Dynamic Light Scattering (DLS), flow cytometry, and Western blotting. These exosomes were then used for loading with S3I-201, resulting in the nano-formulation WJ-Exo(S3I-201). The effect of WJ-Exo(S3I-201) on 4T1 cancer cells was investigated in vitro using MTT assay, flow cytometry, wound healing assay, Western blotting and Quantitative Real-Time Polymerase chain reaction (qPCR) analysis. Finally, the therapeutic efficacy of the nano-formulation was investigated in vivo using a tumor-bearing mouse model.
Results: In vitro experiments showed that co-incubation of 4T1 cells with the nano-formulation resulted in a significant reduction in p-STAT3 levels, induction of apoptosis, modulation of Bcl-2, Bax and caspase-3 protein and gene expression, and inhibition of migration. In vivo, treatment of tumor-bearing mice with WJ-Exo(S3I-201) showed a strong antitumor effect that exceeded the efficacy observed in the S3I-201 group.
Conclusion: Our results demonstrate that WJ-Exo is an effective carrier for targeting S3I-201 to tumor cells and enhances the therapeutic efficacy of S3I-201 in tumor-bearing mice.
Keywords: Breast cancer; Drug delivery; Exosome; STAT3 inhibitor; WJ-mesenchymal stem cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Cetinkaya BD. Targeting the SH2 Domain of STAT3 Proteins in Breast Cancer Treatment. Current Researches in Health Sciences-II, 2023: p. 143.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

