Remifentanil vs. dexmedetomidine for cardiac surgery patients with noninvasive ventilation intolerance: a multicenter randomized controlled trial
- PMID: 39294818
- PMCID: PMC11409483
- DOI: 10.1186/s40560-024-00750-2
Remifentanil vs. dexmedetomidine for cardiac surgery patients with noninvasive ventilation intolerance: a multicenter randomized controlled trial
Abstract
Background: The optimal sedative regime for noninvasive ventilation (NIV) intolerance remains uncertain. The present study aimed to assess the efficacy and safety of remifentanil (REM) compared to dexmedetomidine (DEX) in cardiac surgery patients with moderate-to-severe intolerance to NIV.
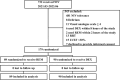
Methods: In this multicenter, prospective, single-blind, randomized controlled study, adult cardiac surgery patients with moderate-to-severe intolerance to NIV were enrolled and randomly assigned to be treated with either REM or DEX for sedation. The status of NIV intolerance was evaluated using a four-point NIV intolerance score at different timepoints within a 72-h period. The primary outcome was the mitigation rate of NIV intolerance following sedation.
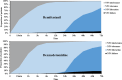
Results: A total of 179 patients were enrolled, with 89 assigned to the REM group and 90 to the DEX group. Baseline characteristics were comparable between the two groups, including NIV intolerance score [3, interquartile range (IQR) 3-3 vs. 3, IQR 3-4, p = 0.180]. The chi-squared test showed that mitigation rate, defined as the proportion of patients who were relieved from their initial intolerance status, was not significant at most timepoints, except for the 15-min timepoint (42% vs. 20%, p = 0.002). However, after considering the time factor, generalized estimating equations showed that the difference was statistically significant, and REM outperformed DEX (odds ratio = 3.31, 95% confidence interval: 1.35-8.12, p = 0.009). Adverse effects, which were not reported in the REM group, were encountered by nine patients in the DEX group, with three instances of bradycardia and six cases of severe hypotension. Secondary outcomes, including NIV failure (5.6% vs. 7.8%, p = 0.564), tracheostomy (1.12% vs. 0%, p = 0.313), ICU LOS (7.7 days, IQR 5.8-12 days vs. 7.0 days, IQR 5-10.6 days, p = 0.219), and in-hospital mortality (1.12% vs. 2.22%, p = 0.567), demonstrated comparability between the two groups.
Conclusions: In summary, our study demonstrated no significant difference between REM and DEX in the percentage of patients who achieved mitigation among cardiac surgery patients with moderate-to-severe NIV intolerance. However, after considering the time factor, REM was significantly superior to DEX. Trial registration ClinicalTrials.gov (NCT04734418), registered on January 22, 2021. URL of the trial registry record: https://register.
Clinicaltrials: gov/prs/app/action/SelectProtocol?sid=S000AM4S&selectaction=Edit&uid=U00038YX&ts=3&cx=eqn1z0 .
Keywords: Cardiac surgery; Dexmedetomidine; Non-invasive ventilation intolerance; Remifentanil.
© 2024. The Author(s).
Conflict of interest statement
None.
Figures

References
-
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med. 2006;32(3):361–70. - PubMed
-
- Hill NS. Where should noninvasive ventilation be delivered? Respir Care. 2009;54(1):62–70. - PubMed
-
- Nava S, Ferrer M, Esquinas A, Scala R, Groff P, Cosentini R, Guido D, Lin CH, Cuomo AM, Grassi M. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol. 2013;14(3):219–27. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

