Efficacy and Safety of Frontline Single-Agent Rituximab in Extranodal Marginal Zone Lymphoma
- PMID: 39295142
- PMCID: PMC11613533
- DOI: 10.1111/ejh.14306
Efficacy and Safety of Frontline Single-Agent Rituximab in Extranodal Marginal Zone Lymphoma
Abstract
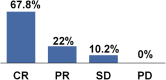
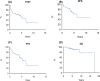
First-line therapy for patients with extranodal marginal zone lymphoma (EMZL) is not well established, except for eradication therapy for Helicobacter pylori in early gastric MZL. Various regimens, for example, locoregional treatment and systemic chemo-immunotherapy, can be used depending on the site and stage of disease. Single-agent rituximab is a useful approach in the setting of localized, low-intermediate risk EMZL. The aim our research was to analyze the effectiveness and safety of single-agent rituximab (375 mg/m2 once weekly for 4 weeks) in naïve EMZL in a real-life setting. The primary endpoint was the overall response rate (ORR), secondary endpoints were progression-free (PFS), overall (OS) and disease-free survivals (DFS), and drug tolerability. Fifty-nine patients were analyzed. Median time between diagnosis and rituximab was 3.6 months. The ORR was 89.9%, with 67.8% complete response (CR). Median DFS and PFS were reached at 6.3 and 5.3 years, respectively. After a median follow-up of 5 years, median OS was not reached. The most common adverse event was infusion reaction, reported in 28 cases, mainly during the first infusion and easily manageable. Single-agent rituximab may represent a valid therapeutic option in the first-line treatment of EMZL, at least for localized disease, with a favorable toxicity profile.
Keywords: extranodal marginal zone lymphoma; frontline treatment; rituximab.
© 2024 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Zinzani P. L. and Broccoli A., “Marginal Zone B‐Cell Lymphomas,” in Williams Hematology (New York, NY: McGraw‐Hill, 2016), 1663–1669.
-
- Chanudet E., Zhou Y., Bacon C. M., et al., “Chlamydia Psittaci Is Variably Associated With Ocular Adnexal MALT Lymphoma in Different Geographical Regions,” Journal of Pathology 209, no. 3 (2006): 344–351. - PubMed
-
- Cerroni L., Zöchling N., Pütz B., and Kerl H., “Infection by Borrelia Burgdorferi and Cutaneous B‐Cell Lymphoma,” Journal of Cutaneous Pathology 24, no. 8 (1997): 457–461. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

