Exploring recent advances in drugs severe cutaneous adverse reactions immunopathology
- PMID: 39295209
- PMCID: PMC11724259
- DOI: 10.1111/all.16316
Exploring recent advances in drugs severe cutaneous adverse reactions immunopathology
Abstract
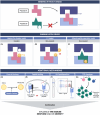
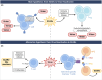
Severe cutaneous adverse reactions to drugs (SCARs) are rare but life-threatening delayed allergies. While they primarily affect the skin, they can also affect internal organs. Accordingly, they present with diverse clinical symptoms that vary not only between SCARs subtypes but also among patients. Despite the availability of topical and systemic treatments, these only address the symptoms and not the cause. To develop more effective therapies, it is necessary to elucidate the complexity of the pathophysiology of SCARs in relation to their severity. In line with the new type IV hypersensitivity reactions nomenclature proposed by the European Academy of Allergy and Clinical Immunology (EAACI), this review highlights the current insights into the intricate immune mechanisms engaged, the interplay between the culprit drug and genetic predisposition in drug presentation mechanisms, but also how external factors, such as viruses, are implicated in SCARs. Their relevance to the development of targeted medicine is also discussed.
Keywords: T‐cell‐mediated diseases; delayed hypersensitivity classification; drug allergy; immunopathology; severe cutaneous adverse reactions.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
All authors report no conflicts of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

