Strategies for minimizing muscle loss during use of incretin-mimetic drugs for treatment of obesity
- PMID: 39295512
- PMCID: PMC11611443
- DOI: 10.1111/obr.13841
Strategies for minimizing muscle loss during use of incretin-mimetic drugs for treatment of obesity
Abstract
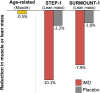
The rapid and widespread clinical adoption of highly effective incretin-mimetic drugs (IMDs), particularly semaglutide and tirzepatide, for the treatment of obesity has outpaced the updating of clinical practice guidelines. Consequently, many patients may be at risk for adverse effects and uncertain long-term outcomes related to the use of these drugs. Of emerging concern is the loss of skeletal muscle mass and function that can accompany rapid substantial weight reduction; such losses can lead to reduced functional and metabolic health, weight cycling, compromised quality of life, and other adverse outcomes. Available evidence suggests that clinical trial participants receiving IMDs for the treatment of obesity lost 10% or more of their muscle mass during the 68- to 72-week interventions, approximately equivalent to 20 years of age-related muscle loss. The ability to maintain muscle mass during caloric restriction-induced weight reduction is influenced by two key factors: nutrition and physical exercise. Nutrition therapy should ensure adequate intake and absorption of high-quality protein and micronutrients, which may require the use of oral nutritional supplements. Additionally, concurrent physical activity, especially resistance training, has been shown to effectively minimize loss of muscle mass and function during weight reduction therapy. All patients receiving IMDs for obesity should participate in comprehensive treatment programs emphasizing adequate protein and micronutrient intakes, as well as resistance training, to preserve muscle mass and function, maximize the benefit of IMD therapy, and minimize potential risks.
Keywords: GLP‐1 receptor agonist; muscle loss; nutrition; resistance training.
© 2024 The Author(s). Obesity Reviews published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Conflict of interest statement
JIM has received honoraria from Abbott Nutrition for lectures and serves on advisory boards for Abbott Nutrition, Aveta.Life, and Twin Health. WSB has received honoraria and/or paid consultancy from Novo Nordisk, Abbott Nutrition, Medscape, Alfie Health, and Med Learning Group. SMC has received honoraria and/or paid consultancy from Novo Nordisk, Eli Lilly, and Abbott Nutrition. OH has received research support from Eli Lilly and Novo Nordisk and serves on an advisory board for Abbott Nutrition. ZL attended the Abbott Nutrition Scientific Roundtable meeting. CMP has received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, Pfizer, and AMRA medical and investigator‐initiated grant funding from Almased. SBH has received honoria/paid consultancy from Medifast Corporation, Abbott Nutrition, Tanita Corporation, Novo Nordisk, Versanis, and Amgen.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

