Dual optical elastography detects -induced alterations in the biomechanical properties of skin scaffolds
- PMID: 39295639
- PMCID: PMC11409821
- DOI: 10.1117/1.JBO.29.9.095002
Dual optical elastography detects -induced alterations in the biomechanical properties of skin scaffolds
Abstract
Significance: The skin's mechanical properties are tightly regulated. Various pathologies can affect skin stiffness, and understanding these changes is a focus in tissue engineering. Ex vivo skin scaffolds are a robust platform for evaluating the effects of various genetic and molecular interactions on the skin. Transforming growth factor-beta ( ) is a critical signaling molecule in the skin that can regulate the amount of collagen and elastin in the skin and, consequently, its mechanical properties.
Aim: This study investigates the biomechanical properties of bio-engineered skin scaffolds, focusing on the influence of , a signaling molecule with diverse cellular functions.
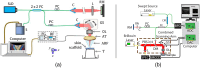
Approach: The receptor I inhibitor, galunisertib, was employed to assess the mechanical changes resulting from dysregulation of . Skin scaffold samples, grouped into three categories (control, -treated, and + galunisertib-treated), were prepared in two distinct culture media-one with aprotinin (AP) and another without. Two optical elastography techniques, namely wave-based optical coherence elastography (OCE) and Brillouin microscopy, were utilized to quantify the biomechanical properties of the tissues.
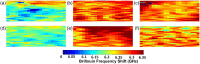
Results: Results showed significantly higher wave speed (with AP, ; without AP, ) and Brillouin frequency shift (with AP, ; without AP, ) in -treated group compared with the control group. The difference in wave speed between the control and + galunisertib with ( ) and without AP ( ) was not significant. Moreover, the + galunisertib-treated group exhibited lower wave speed without and with AP and reduced Brillouin frequency shift than the -treated group without AP, further strengthening the potential role of in regulating the mechanical properties of the samples.
Conclusions: These findings offer valuable insights into -induced biomechanical alterations in bio-engineered skin scaffolds, highlighting the potential of OCE and Brillouin microscopy in the development of targeted therapies in conditions involving abnormal tissue remodeling and fibrosis.
Keywords: Brillouin microscopy; bioengineered skin; elasticity; optical coherence elastography; tissue scaffold.
© 2024 The Authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

