Assessing Comparative Efficacy of Biologics for the Treatment of Psoriasis With Nail Involvement: A Systematic Review
- PMID: 39295894
- PMCID: PMC11361496
- DOI: 10.1177/24755303231217491
Assessing Comparative Efficacy of Biologics for the Treatment of Psoriasis With Nail Involvement: A Systematic Review
Abstract
Background: Despite recent advances in biologics, there is a lack of significant evidence regarding the comparative efficacy of biologics in treating more resistant features of psoriasis, namely nail psoriasis. A systematic review synthesizing data from multiple studies is efficacious in assessing the comparative efficacy among biologics for the treatment of nail psoriasis.
Objective: To evaluate and compare the efficacy of biologics for the treatment of nail psoriasis.
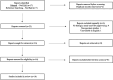
Methods: Utilizing PRISMA guidelines, a systematic literature review was conducted using the Pubmed database on November 16, 2022. Studies selected were phase 3 or 4 randomized clinical trials, clinical studies, or other randomized trials with data on the treatment with biologics for adults with nail psoriasis.
Results: Sixteen studies meeting inclusion criteria were included for analysis. At 24 weeks, the highest mean NAPSI percent improvement achieved at week 24 was by brodalumab (76.9%) followed by etanercept (74%) and ixekizumab (70.5%) while the biologics achieving the greatest proportion of NAPSI 0 were adalimumab (44.6%) and ixekizumab (41%).
Conclusions: This study helps elucidate the comparative efficacy of biologics for the treatment of nail psoriasis. This review suggests that brodalumab and etanercept are associated with the highest percent improvement in nail psoriasis while adalimumab and ixekizumab are associated with the greatest probability of complete nail resolution.
Keywords: biologics; nail psoriasis; psoriasis; psoriatic nail changes.
© The Author(s) 2023.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Similar articles
-
Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis.J Dermatolog Treat. 2022 May;33(3):1652-1660. doi: 10.1080/09546634.2021.1892024. Epub 2021 Mar 1. J Dermatolog Treat. 2022. PMID: 33641593
-
Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24-28 and 48-52 weeks.J Dermatolog Treat. 2023 Dec;34(1):2263108. doi: 10.1080/09546634.2023.2263108. Epub 2023 Oct 2. J Dermatolog Treat. 2023. PMID: 37781881
-
Simultaneous Nail and Skin Clearance in Ixekizumab Head-to-Head Trials for Moderate-to-Severe Psoriasis and Psoriatic Arthritis.Dermatol Ther (Heidelb). 2022 Apr;12(4):911-920. doi: 10.1007/s13555-022-00704-2. Epub 2022 Mar 13. Dermatol Ther (Heidelb). 2022. PMID: 35279805 Free PMC article.
-
Efficacy and Safety of Nail Psoriasis Targeted Therapies: A Systematic Review.Am J Clin Dermatol. 2023 Sep;24(5):695-720. doi: 10.1007/s40257-023-00786-4. Epub 2023 May 20. Am J Clin Dermatol. 2023. PMID: 37209391
-
Nail psoriasis and biologics.J Cutan Med Surg. 2009 Jan-Feb;13(1):1-5. doi: 10.2310/7750.2008.08012. J Cutan Med Surg. 2009. PMID: 19298765 Review.
References
-
- Cassell SE, Bieber JD, Rich P, et al. The modified nail psoriasis severity index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol. 2007;34:123-129. - PubMed
-
- Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79:123-131. doi:10.1136/annrheumdis-2019-215386. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources