Paulownin elicits anti-tumor effects by enhancing NK cell cytotoxicity through JNK pathway activation
- PMID: 39295927
- PMCID: PMC11408334
- DOI: 10.3389/fphar.2024.1439079
Paulownin elicits anti-tumor effects by enhancing NK cell cytotoxicity through JNK pathway activation
Abstract
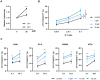
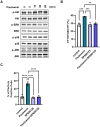
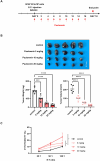
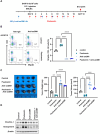
Paulownin, a natural compound derived from Paulownia tomentosa wood, exhibits various physiological functions, including anti-bacterial and anti-fungal effects. However, the impact of paulownin on natural killer (NK) cell immune activity remains largely unknown. In this study, we investigated the effect of paulownin on NK cell activity both in vitro and in vivo, and explored its potential mechanisms. NK-92 cells were used for in vitro experiments and a BALB/c mouse model with B16F10 cells injected subcutaneously were used for in vivo anti-tumor analysis. We found that paulownin enhanced the cytolytic activity of NK-92 cells against leukemia, human colon, and human lung cancer cell lines. Paulownin treatment increased the expression of the degranulation marker protein CD107a and cytolytic granules, including granzyme B and perforin in NK-92 cells. Moreover, these enhancements of cytotoxicity and the expression of cytolytic granules induced by paulownin were also observed in human primary NK cells. Signaling studies showed that paulownin promoted the phosphorylation of JNK. The increased perforin expression and elevated cytotoxic activity induced by paulownin were effectively inhibited by pre-treatment with a JNK inhibitor. In vivo studies demonstrated that the administration of paulownin suppressed the growth of B16F10 melanoma cells allografted into mice. Paulownin administration promoted the activation of NK cells in the spleen of mice, resulting in enhanced cytotoxicity against YAC-1 cells. Moreover, the anti-tumor effects of paulownin were reduced upon the depletion of NK cells. Therefore, these results suggest that paulownin enhances NK cell cytotoxicity by activating the JNK signaling pathway and provide significant implications for developing new strategies for cancer immunotherapy.
Keywords: JNK; NK cells; immunotherapy; innate immunity; paulownin.
Copyright © 2024 Park, Hwang, Ryu, Yoon, Kim, Lim, Cho and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Chen X., Jin J., Hao F., Yang H., Sun H., Jiang C. (2021). Paulownia tomentosa flower polysaccharide as an effective immunopotentiator to enhance immune responses for Newcastle disease vaccine in mice. Italian J. Food Sci. 33, 11–20. 10.15586/ijfs.v33i4.2107 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

