Impact of hepatitis B immunoglobulin mode of administration on treatment experiences of patients after liver transplantation: Results from an online survey
- PMID: 39295979
- PMCID: PMC11317858
- DOI: 10.5500/wjt.v14.i3.90949
Impact of hepatitis B immunoglobulin mode of administration on treatment experiences of patients after liver transplantation: Results from an online survey
Abstract
Background: Hepatitis B immunoglobulin (HBIG) in combination with a potent nucleos(t)ide analog is considered the standard of care for prophylaxis against hepatitis B virus (HBV) reinfection after liver transplantation for HBV-associated disease.
Aim: To evaluate patients' satisfaction, preferences, and requirements for subcutaneous (SC), intramuscular (IM), and intravenous (IV) HBIG treatments.
Methods: A self-completion, cross-sectional, online, 22-question survey was conducted to examine perceptions and satisfaction with current HBIG treatment in adults receiving HBIG treatment following liver transplantation for HBV-associated disease in France, Italy, and Turkey. Hypothetical HBIG products with different administration modes were evaluated using target product profile assessment and a conjoint (trade-off) exercise.
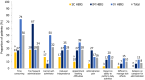
Results: Ninety patients were enrolled; 32%, 17%, and 51% were SC, IM, and IV HBIG users, respectively. Mean duration of treatment was 36.2 months. SC HBIG had the least negative impact on emotional well-being and social life and was perceived as the most convenient, easiest to administer, least painful, and had the highest self-rating of treatment compliance. More IM HBIG users than SC or IV HBIG users reported that administration frequency was excessive (67%, 28%, and 28%, respectively). In the target product profile assessment, 76% of patients were likely to use hypothetical SC HBIG. In the conjoint exercise, administration route, frequency, and duration were key drivers of treatment preferences.
Conclusion: Ease, frequency, duration, and side effects of HBIG treatment administration were key drivers of treatment preferences, and SC HBIG appeared advantageous over IM and IV HBIG for administration ease, convenience, and pain. A hypothetical SC HBIG product elicited a favorable response. Patient demographics, personal preferences, and satisfaction with HBIG treatment modalities may influence long-term treatment compliance.
Keywords: Hepatitis B immunoglobulin; Intramuscular; Intravenous; Liver transplantation; Patient satisfaction; Subcutaneous.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Glynou K is an employee of Biotest AG. Eletskaya M is an employee of Lumanity (previously Cello Health Insight); the study was conducted under a contract with Biotest AG. Rizza G received consulting fees under a contract with Biotest AG. The authors have no non-financial competing interests to declare.
Figures




References
-
- Dutkowski P, Linecker M, DeOliveira ML, Müllhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307–323. - PubMed
-
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
-
- British Transplantation Society. Guidelines for hepatitis B & solid organ transplantation. 2018. [cited 20 December 2023]. Available from: https://bts.org.uk/wp-content/uploads/2018/01/BTS-Hep-B-Guidelines-26-Ja... .
LinkOut - more resources
Full Text Sources

