Design, Synthesis, and In Vitro Characterization of Proteolytically-Stable Opioid-Neurotensin Hybrid Peptidomimetics
- PMID: 39296263
- PMCID: PMC11406707
- DOI: 10.1021/acsptsci.4c00236
Design, Synthesis, and In Vitro Characterization of Proteolytically-Stable Opioid-Neurotensin Hybrid Peptidomimetics
Abstract
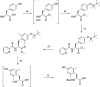
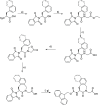
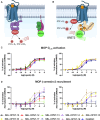
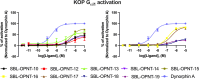
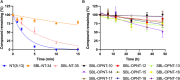
Linking an opioid to a nonopioid pharmacophore represents a promising approach for reducing opioid-induced side effects during pain management. Herein, we describe the optimization of the previously reported opioid-neurotensin hybrids (OPNT-hybrids), SBL-OPNT-05 & -10, containing the μ-/δ-opioid agonist H-Dmt-d-Arg-Aba-β-Ala-NH2 and NT(8-13) analogs optimized for NTS2 affinity. In the present work, the constrained dipeptide Aba-β-Ala was modified to investigate the optimal linker length between the two pharmacophores, as well as the effect of expanding the aromatic moiety within constrained dipeptide analogs, via the inclusion of a naphthyl moiety. Additionally, the N-terminal Arg residue of the NT(8-13) pharmacophore was substituted with β3 hArg. For all analogs, affinity was determined at the MOP, DOP, NTS1, and NTS2 receptors. Several of the hybrid ligands showed a subnanomolar affinity for MOP, improved binding for DOP compared to SBL-OPNT-05 & -10, as well as an excellent NTS2-affinity with high selectivity over NTS1. Subsequently, the Gαi1 and β-arrestin-2 pathways were evaluated for all hybrids, along with their stability in rat plasma. Upon MOP activation, SBL-OPNT-13 and -18 were the least effective at recruiting β-arrestin-2 (E max = 17 and 12%, respectively), while both compounds were also found to be partial agonists at the Gαi1 pathway, despite improved potency compared to DAMGO. Importantly, these analogs also showed a half-life in rat plasma in excess of 48 h, making them valuable tools for future in vivo investigations.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- DeWire S. M.; Yamashita D. S.; Rominger D. H.; Liu G.; Cowan C. L.; Graczyk T. M.; Chen X.-T.; Pitis P. M.; Gotchev D.; Yuan C.; Koblish M.; Lark M. W.; Violin J. D. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 2013, 344 (3), 708–717. 10.1124/jpet.112.201616. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
