Withaferin A Ameliorated the Bone Marrow Fat Content in Obese Male Mice by Favoring Osteogenesis in Bone Marrow Mesenchymal Stem Cells and Preserving the Bone Mineral Density
- PMID: 39296264
- PMCID: PMC11406682
- DOI: 10.1021/acsptsci.3c00356
Withaferin A Ameliorated the Bone Marrow Fat Content in Obese Male Mice by Favoring Osteogenesis in Bone Marrow Mesenchymal Stem Cells and Preserving the Bone Mineral Density
Abstract
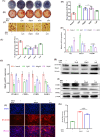
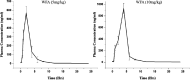
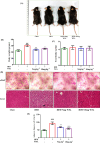
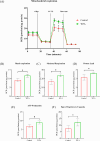
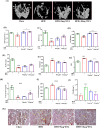
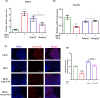
Obesity and osteoporosis are two prevalent conditions that are becoming increasingly common worldwide, primarily due to aging populations, imbalanced energy intake, and sedentary lifestyles. Obesity, characterized by excessive fat accumulation, and osteoporosis, marked by reduced bone density and increased fracture risk, are often interconnected. High-fat diets (HFDs) can exacerbate both conditions by promoting bone marrow adiposity and bone loss. The effect of WFA on the osteogenesis and adipogenesis was studied on the C3H10T1/2 cell line and bone marrow mesenchymal stem cells (BM-MSCs) isolated from mice. We used oil red O and alkaline phosphatase (ALP) staining to observe adipogenesis and osteogenesis, respectively, in MSCs. Real-time PCR and Western blot analyses were used to study the molecular effects of WFA on MSCs. We employed micro-CT to analyze the bone microarchitecture, bone mineral density (BMD), and abdominal fat mass in male mice. We have used osmium tetroxide (OsO4) staining to study the bone marrow fat. WFA induced the C3H10T1/2 cell line and BM-MSCs toward osteogenic lineage as evidenced by the higher ALP activity. WFA also downregulated the lipid droplet formation and adipocyte specific genes in MSCs. In the in vivo study, WFA also suppressed the bone catabolic effects of the HFD and maintained the bone microarchitecture and BMD in WFA-treated animals. The bone marrow adipose tissue was reduced in the tibia of WFA-treated groups in comparison with only HFD-fed animals. Withaferin A was able to improve the bone microarchitecture and BMD by committing BM-MSCs toward osteogenic differentiation and reducing marrow adiposity. The findings of this study could provide valuable insights into the therapeutic potential of Withaferin A for combating bone marrow obesity and osteoporosis, particularly in the context of diet-induced metabolic disturbances.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Gyllenhammer L. E.; Alderete T. L.; Toledo-Corral C. M.; Weigensberg M.; Goran M. I. Saturation of Subcutaneous Adipose Tissue Expansion and Accumulation of Ectopic Fat Associated with Metabolic Dysfunction during Late and Post-Pubertal Growth. Int. J. Obes (Lond) 2016, 40 (4), 601–606. 10.1038/ijo.2015.207. - DOI - PMC - PubMed
-
- Kranioti E. F.; Bonicelli A.; García-Donas J. G. Bone-Mineral Density: Clinical Significance, Methods of Quantification and Forensic Applications. Res. Rep. Forensic Med. Sci. 2019, 9, 9–21. 10.2147/RRFMS.S164933. - DOI
-
- Griffith J. F.; Yeung D. K. W.; Antonio G. E.; Lee F. K. H.; Hong A. W. L.; Wong S. Y. S.; Lau E. M. C.; Leung P. C. Vertebral Bone Mineral Density, Marrow Perfusion, and Fat Content in Healthy Men and Men with Osteoporosis: Dynamic Contrast-Enhanced MR Imaging and MR Spectroscopy. Radiology 2005, 236 (3), 945–951. 10.1148/radiol.2363041425. - DOI - PubMed
LinkOut - more resources
Full Text Sources
