SARS-CoV-2 replicates in the placenta after maternal infection during pregnancy
- PMID: 39296889
- PMCID: PMC11409086
- DOI: 10.3389/fmed.2024.1439181
SARS-CoV-2 replicates in the placenta after maternal infection during pregnancy
Abstract
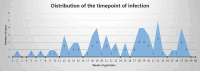
Objectives: Pregnant women are at increased risk for severe SARS-CoV-2 infection and adverse neonatal outcome, primarily preterm birth and stillbirth. Our study aimed to investigate to which extent SARS-CoV-2 affects placental tissue and if viral replication within the placenta is evident, thus if there is a correlation between placental damage and adverse pregnancy outcome such as stillbirth.
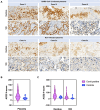
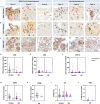
Methods: We prospectively collected placentas from 61 SARS-CoV-2 infected pregnant women and 10 controls. Histopathological, immunohistochemical, and in situ hybridization studies were performed on all placentas with antibodies for SARS-CoV-2 proteins, ACE2, various immune cells, and inflammatory markers or probes for SARS-CoV-2 genes and an antisense strand.
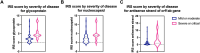
Results: The measured scores of SARS-CoV-2 glycoprotein, nucleocapsid, and antisense strand indicating replication correlated with both the severity of maternal symptoms and presence of stillbirth. Specifically, 15/61 placentas exhibited replication, while the three cases with stillbirth had high or maximal replication scores. ACE2-H-score was significantly higher in COVID-19 patients, while the expression of various immune cells did not differ statistically. In multivariate analysis, presence of maternal comorbidities correlated with presence of severe COVID-19 infection.
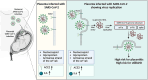
Conclusion: We report evidence of active in vivo SARS-CoV-2 replication in the placenta after maternal infection in pregnancy in a case-control setting in a large population. Intensity of placental viral replication as well as viral levels were higher in women with severe or critical COVID-19 disease, supporting the rationale that severity of maternal SARS-CoV-2 infection could correlate with the severity of placentitis. Replication was maximal in cases of stillbirth, which suggests direct placental involvement in the pathophysiology of this dramatic outcome. Continuing to advocate for preventive measures against COVID-19 during pregnancy, including (re)vaccination, as well as appropriately counseling women with diagnosed infection, are of utter importance.
Keywords: COVID-19; SARS-CoV-2; SARS-CoV-2 replication; placenta; stillbirth.
Copyright © 2024 Radan, Renz, Raio, Villiger, Haesler, Trippel and Surbek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

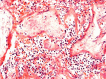
References
-
- Gurol-Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. (2021) 225:522.e1–522.e11. doi: 10.1016/j.ajog.2021.05.016, PMID: - DOI - PMC - PubMed
-
- Roberts DJ, Edlow AG, Romero RJ, Coyne CB, Ting DT, Hornick JL, et al. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS-CoV-2 placental infection workshop. Am J Obstet Gynecol. (2021) 225:593.e1–9. doi: 10.1016/j.ajog.2021.07.029, PMID: - DOI - PMC - PubMed
-
- Stenton S, McPartland J, Shukla R, Turner K, Marton T, Hargitai B, et al. SARS-COV2 placentitis and pregnancy outcome: a multicentre experience during the alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine. (2022) 47:101389. doi: 10.1016/j.eclinm.2022.101389, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

