The Neurostimulationist will see you now: prescribing direct electrical stimulation therapies for the human brain in epilepsy and beyond
- PMID: 39296917
- PMCID: PMC11408201
- DOI: 10.3389/fnhum.2024.1439541
The Neurostimulationist will see you now: prescribing direct electrical stimulation therapies for the human brain in epilepsy and beyond
Abstract
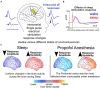
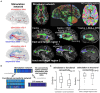
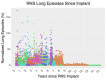
As the pace of research in implantable neurotechnology increases, it is important to take a step back and see if the promise lives up to our intentions. While direct electrical stimulation applied intracranially has been used for the treatment of various neurological disorders, such as Parkinson's, epilepsy, clinical depression, and Obsessive-compulsive disorder, the effectiveness can be highly variable. One perspective is that the inability to consistently treat these neurological disorders in a standardized way is due to multiple, interlaced factors, including stimulation parameters, location, and differences in underlying network connectivity, leading to a trial-and-error stimulation approach in the clinic. An alternate view, based on a growing knowledge from neural data, is that variability in this input (stimulation) and output (brain response) relationship may be more predictable and amenable to standardization, personalization, and, ultimately, therapeutic implementation. In this review, we assert that the future of human brain neurostimulation, via direct electrical stimulation, rests on deploying standardized, constrained models for easier clinical implementation and informed by intracranial data sets, such that diverse, individualized therapeutic parameters can efficiently produce similar, robust, positive outcomes for many patients closer to a prescriptive model. We address the pathway needed to arrive at this future by addressing three questions, namely: (1) why aren't we already at this prescriptive future?; (2) how do we get there?; (3) how far are we from this Neurostimulationist prescriptive future? We first posit that there are limited and predictable ways, constrained by underlying networks, for direct electrical stimulation to induce changes in the brain based on past literature. We then address how identifying underlying individual structural and functional brain connectivity which shape these standard responses enable targeted and personalized neuromodulation, bolstered through large-scale efforts, including machine learning techniques, to map and reverse engineer these input-output relationships to produce a good outcome and better identify underlying mechanisms. This understanding will not only be a major advance in enabling intelligent and informed design of neuromodulatory therapeutic tools for a wide variety of neurological diseases, but a shift in how we can predictably, and therapeutically, prescribe stimulation treatments the human brain.
Keywords: direct electrical stimulation; epilepsy; human; intracranial; neural activity.
Copyright © 2024 Hadar, Zelmann, Salami, Cash and Paulk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Albert G. C., Cook C. M., Prato F. S., Thomas A. W. (2009). Deep brain stimulation, vagal nerve stimulation and transcranial stimulation: an overview of stimulation parameters and neurotransmitter release. Neurosci. Biobehav. Rev. 33, 1042–1060. doi: 10.1016/j.neubiorev.2009.04.006, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

