Tongue exercise ameliorates structural and functional upper airway deficits in a rodent model of hypoglossal motor neuron loss
- PMID: 39296960
- PMCID: PMC11408480
- DOI: 10.3389/fneur.2024.1441529
Tongue exercise ameliorates structural and functional upper airway deficits in a rodent model of hypoglossal motor neuron loss
Abstract
Introduction: Tongue weakness and atrophy can lead to deficits in the vital functions of breathing and swallowing in patients with motor neuron diseases (MNDs; e.g., amyotrophic lateral sclerosis (ALS) and pseudobulbar palsy), often resulting in aspiration pneumonia, respiratory failure, and death. Available treatments for patients with MNDs are largely palliative; thus, there is a critical need for therapies targeting preservation of upper airway function and suggesting a role for tongue exercise in patients with MNDs. Here, we leveraged our inducible rodent model of hypoglossal (XII) motor neuron degeneration to investigate the effects of a strength endurance tongue exercise program on upper airway structure and function. Our model was created through intralingual injection of cholera toxin B conjugated to saporin (CTB-SAP) into the genioglossus muscle of the tongue to induce targeted death of XII motor neurons.
Methods: Rats in this study were allocated to 4 experimental groups that received intralingual injection of either CTB-SAP or unconjugated CTB + SAP (i.e., control) +/- tongue exercise. Following tongue exercise exposure, we evaluated the effect on respiratory function (via plethysmography), macrostructure [via magnetic resonance imaging (MRI) of the upper airway and tongue], and ultrafine structure [via ex vivo magnetic resonance spectroscopy (MRS) of the tongue] with a focus on lipid profiles.
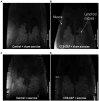
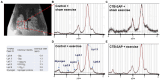
Results: Results showed that sham exercise-treated CTB-SAP rats have evidence of upper airway restriction (i.e., reduced airflow) and structural changes present in the upper airway (i.e., airway compression) when compared to CTB-SAP + exercise rats and control rats +/- tongue exercise, which was ameliorated with tongue exercise. Additionally, CTB-SAP + sham exercise rats have evidence of increased lipid expression in the tongue consistent with previously observed tongue hypertrophy when compared to CTB-SAP + exercise rats or control rats +/- tongue exercise.
Conclusion: These findings provide further evidence that a strength endurance tongue exercise program may be a viable therapeutic treatment option in patients with XII motor neuron degeneration in MNDs such as ALS. Future directions will focus on investigating the underlying mechanism responsible for tongue exercise-induced plasticity in the hypoglossal-tongue axis, particularly inflammatory associated factors such as BDNF.
Keywords: breathing; degeneration; dysphagia; motor neuron disease (MND); rat model; respiration.
Copyright © 2024 Keilholz, Pathak, Smith, Osman, Smith, Oti, Golzy, Ma, Lever and Nichols.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

