Cell Membrane-Coated Nanoparticles for Dental, Oral, and Craniofacial Diseases
- PMID: 39296987
- PMCID: PMC11409001
- DOI: 10.34133/research.0478
Cell Membrane-Coated Nanoparticles for Dental, Oral, and Craniofacial Diseases
Abstract
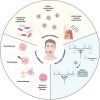
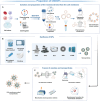
Dental, oral, and craniofacial diseases can substantially impact the quality of human life, thereby posing a serious public health concern. Although conventional therapies such as surgery have solved these problems largely, the prognosis of patients is not always satisfactory. Cell membrane-coated nanoparticles (CMCNPs) carry nanodrugs with the help of natural cell membranes, therefore utilizing their remarkable ability to interface and interact with their surrounding environment. These nanoparticles have demonstrated substantial advantages in drug targeting, prolonging blood circulation time, penetrating biofilms, and immune escape. With the assistance of CMCNPs, the therapeutic effects of dental, oral, and craniofacial diseases can reach a higher level. CMCNPs have been applied for dental, oral, and craniofacial diseases for various conditions such as head and neck cancer, periodontal disease, and oral biosignal detection. For the therapies of head and neck cancer, CMCNPs have been widely utilized as a tool of chemotherapy, phototherapy, and immunotherapy, while yet to be exploited in imaging technique. In the end, we summarized the challenges and prospectives of CMCNPs for dental, oral, and craniofacial diseases: large-scale production with uniform standards and high quantity, extensive application directions in dental, oral, and craniofacial regions (implant, endodontics), and the promotion of its clinical application.
Copyright © 2024 Kang-Ning Wang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures






References
-
- Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, Kearns C, et al. Oral diseases: A global public health challenge. Lancet. 2019;394(10194):249–260. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. - PubMed
-
- Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 2017;44(5):456–462. - PubMed
-
- Slots J. Periodontitis: Facts, fallacies and the future. Periodontol 2000. 2017;75(1):7–23. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

