Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles
- PMID: 39297017
- PMCID: PMC11408389
- DOI: 10.1016/j.ijpx.2024.100281
Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles
Abstract
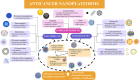
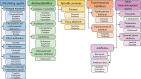
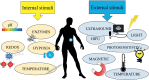
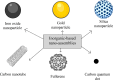
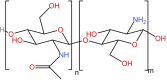
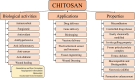
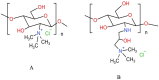
Cancer is the leading cause of death globally, and conventional treatments have limited efficacy with severe side effects. The use of nanotechnology has the potential to reduce the side effects of drugs by creating efficient and controlled anticancer drug delivery systems. Nanoparticles (NPs) used as drug carriers offer several advantages, including enhanced drug protection, biodistribution, selectivity and, pharmacokinetics. Therefore, this review is devoted to various organic (lipid, polymeric) as well as inorganic nanoparticles based on different building units and providing a wide range of potent anticancer drug delivery systems. Within these nanoparticulate systems, chitosan (CS)-based NPs are discussed with particular emphasis due to the unique properties of CS and its derivatives including non-toxicity, biodegradability, mucoadhesivity, and tunable physico-chemical as well as biological properties allowing their alteration to specifically target cancer cells. In the context of streamlining the nanoparticulate drug delivery systems (DDS), innovative nanoplatform-based cancer therapy pathways involving passive and active targeting as well as stimuli-responsive DDS enhancing overall orthogonality of developed NP-DDS towards the target are included. The most up-to-date information on delivering anti-cancer drugs using modern dosage forms based on various nanoparticulate systems and, specifically, CSNPs, are summarised and evaluated concerning their benefits, limitations, and advanced applications.
Keywords: Anticancer drugs; Chitosan; Chitosan derivatives; Lipid and polymeric nanoparticles; Nanoparticulate drug delivery systems; Organic and inorganic nanoparticles.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
None.
Figures











References
-
- Afra S., Samadi A., Asadi P., Bordbar M., Iloukhani M., Rai A., Aghajanpour M. Chitosan crosslinkers and their functionality in 3D bioprinting to produce chitosan-based bioinks. Inorg. Chem. Commun. 2024;168 doi: 10.1016/j.inoche.2024.112842. - DOI
-
- Aguanell A., del Pozo M.L., Pérez-Martín C., Pontes G., Bastida A., Fernández-Mayoralas A., García-Junceda E., Revuelta J. Chitosan sulfate-lysozyme hybrid hydrogels as platforms with fine-tuned degradability and sustained inherent antibiotic and antioxidant activities. Carbohydr. Polym. 2022;291 doi: 10.1016/j.carbpol.2022.119611. - DOI - PubMed
-
- Ahmadi F., Sodagar-Taleghani A., Ebrahimnejad P., Pouya Hadipour Moghaddam S., Ebrahimnejad F., Asare-Addo K., Nokhodchi A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022;625 doi: 10.1016/j.ijpharm.2022.122099. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

