A New Ex Vivo Model to Study Cardiac Fibrosis in Whole Mouse Hearts
- PMID: 39297130
- PMCID: PMC11405901
- DOI: 10.1016/j.jacbts.2024.04.007
A New Ex Vivo Model to Study Cardiac Fibrosis in Whole Mouse Hearts
Abstract
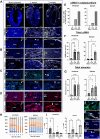
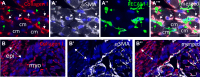
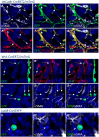
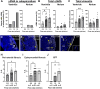
Fibrosis is a characteristic of many cardiac diseases for which no effective treatment exists. We have developed an ex vivo flow system, which allows induction of cardiac fibrosis in intact adult mouse hearts. Lineage-tracing studies indicated that the collagen-producing myofibroblasts originated from the resident fibroblasts. The extent of fibrosis was flow rate dependent, and pharmacological inhibition of the transforming growth factor beta signaling pathway prevented fibrosis. Therefore, in this powerful system, the cellular and molecular mechanisms underlying cardiac fibrosis can be studied. In addition, new targets can be tested on organ level for their ability to inhibit fibrosis.
Keywords: MTCS; TGFbeta; flow model; mechanical environment; preclinical translational.
© 2024 The Authors.
Conflict of interest statement
This project has received funding form the Ministry of Economic Affairs under TKI-Allowance under the TKI-programme Life Sciences & Health and the Dutch Heart Foundation / Hartstichting. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



Similar articles
-
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article.
-
MicroRNA‑331 inhibits isoproterenol‑induced expression of profibrotic genes in cardiac myofibroblasts via the TGFβ/smad3 signaling pathway.Sci Rep. 2021 Jan 28;11(1):2548. doi: 10.1038/s41598-021-82226-z. Sci Rep. 2021. PMID: 33510328 Free PMC article.
-
Apilimod alters TGFβ signaling pathway and prevents cardiac fibrotic remodeling.Theranostics. 2021 Apr 19;11(13):6491-6506. doi: 10.7150/thno.55821. eCollection 2021. Theranostics. 2021. PMID: 33995670 Free PMC article.
-
Novel factors that activate and deactivate cardiac fibroblasts: A new perspective for treatment of cardiac fibrosis.Wound Repair Regen. 2021 Jul;29(4):667-677. doi: 10.1111/wrr.12947. Epub 2021 Jun 16. Wound Repair Regen. 2021. PMID: 34076932 Review.
-
Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast.Curr Med Chem. 2009;16(11):1400-17. doi: 10.2174/092986709787846497. Curr Med Chem. 2009. PMID: 19355895 Review.
Cited by
-
Cryopreservation of Human Adult Ventricular Tissue for the Preparation of Viable Myocardial Slices.Curr Protoc. 2024 Dec;4(12):e70068. doi: 10.1002/cpz1.70068. Curr Protoc. 2024. PMID: 39625241 Free PMC article.
-
Wilms tumor 1 impairs apoptotic clearance of fibroblasts in distal fibrotic lung lesions.J Clin Invest. 2025 Jun 10;135(15):e188819. doi: 10.1172/JCI188819. eCollection 2025 Aug 1. J Clin Invest. 2025. PMID: 40493404 Free PMC article.
References
-
- Diez J., de Boer R.A. Management of cardiac fibrosis is the largest unmet medical need in heart failure. Cardiovasc Res. 2022;118:e20–e22. - PubMed
-
- Frangogiannis N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. - PubMed
-
- Frangogiannis N.G. Transforming growth factor-beta in myocardial disease. Nat Rev Cardiol. 2022;19:435–455. - PubMed
LinkOut - more resources
Full Text Sources

