Carvedilol Activates a Myofilament Signaling Circuitry to Restore Cardiac Contractility in Heart Failure
- PMID: 39297139
- PMCID: PMC11405995
- DOI: 10.1016/j.jacbts.2024.03.007
Carvedilol Activates a Myofilament Signaling Circuitry to Restore Cardiac Contractility in Heart Failure
Abstract
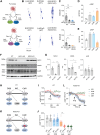
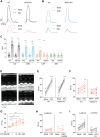
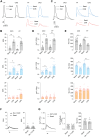
Phosphorylation of myofilament proteins critically regulates beat-to-beat cardiac contraction and is typically altered in heart failure (HF). β-Adrenergic activation induces phosphorylation in numerous substrates at the myofilament. Nevertheless, how cardiac β-adrenoceptors (βARs) signal to the myofilament in healthy and diseased hearts remains poorly understood. The aim of this study was to uncover the spatiotemporal regulation of local βAR signaling at the myofilament and thus identify a potential therapeutic target for HF. Phosphoproteomic analysis of substrate phosphorylation induced by different βAR ligands in mouse hearts was performed. Genetically encoded biosensors were used to characterize cyclic adenosine and guanosine monophosphate signaling and the impacts on excitation-contraction coupling induced by β1AR ligands at both the cardiomyocyte and whole-heart levels. Myofilament signaling circuitry was identified, including protein kinase G1 (PKG1)-dependent phosphorylation of myosin light chain kinase, myosin phosphatase target subunit 1, and myosin light chain at the myofilaments. The increased phosphorylation of myosin light chain enhances cardiac contractility, with a minimal increase in calcium (Ca2+) cycling. This myofilament signaling paradigm is promoted by carvedilol-induced β1AR-nitric oxide synthetase 3 (NOS3)-dependent cyclic guanosine monophosphate signaling, drawing a parallel to the β1AR-cyclic adenosine monophosphate-protein kinase A pathway. In patients with HF and a mouse HF model of myocardial infarction, increasing expression and association of NOS3 with β1AR were observed. Stimulating β1AR-NOS3-PKG1 signaling increased cardiac contraction in the mouse HF model. This research has characterized myofilament β1AR-PKG1-dependent signaling circuitry to increase phosphorylation of myosin light chain and enhance cardiac contractility, with a minimal increase in Ca2+ cycling. The present findings raise the possibility of targeting this myofilament signaling circuitry for treatment of patients with HF.
Keywords: contractility; heart failure; myofilament; myosin light chain; nitric oxide synthetase; protein kinase G; β1-adrenoceptor.
© 2024 The Authors.
Conflict of interest statement
This work was supported by National Institutes of Health grants R01-HL147263 and HL162825, VA Merit grants IK6BX005753 and BX005100 (to Dr Xiang). Drs Wang and Zhu are recipients of American Heart Association postdoctoral fellowship. Dr Xiang is an established American Heart Association investigator. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

