3D Printed Mesh Geometry Modulates Immune Response and Interface Biology in Mouse and Sheep Model: Implications for Pelvic Floor Surgery
- PMID: 39297316
- PMCID: PMC11923936
- DOI: 10.1002/advs.202405004
3D Printed Mesh Geometry Modulates Immune Response and Interface Biology in Mouse and Sheep Model: Implications for Pelvic Floor Surgery
Abstract
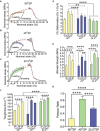
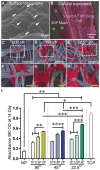
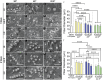
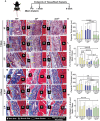
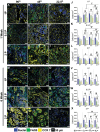
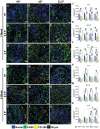
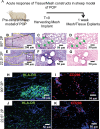
Pelvic organ prolapse (POP) is a highly prevalent yet neglected health burden for women. Strengthening the pelvic floor with bioactive tissue-engineered meshes is an emerging concept. This study investigates tissue regenerative design parameters, including degradability, porosity, and angulation, to develop alternative degradable melt electrowritten (MEW) constructs for surgical applications of POP. MEW constructs were fabricated in hierarchical geometries by two-way stacking of the fibers with three different inter layer angles of 90°, 45°, or 22.5°. Implants printed at 22.5° have higher tensile strength under dry conditions and show better vaginal fibroblast (VF) attachment in vitro. In vivo assessment using preclinical mouse and ovine models demonstrates more effective degradation and improved tissue integration in 22.5° angular meshes compared to 90° and 45° meshes, with evidence of neo-collagen deposition within implants at 6 weeks. The pattern and geometry of the layered MEW implants also influence the foreign body response, wherein the anti-inflammatory phenotype shows a greater ratio of anti-inflammatory CD206+ M2 macrophages/pro-inflammatory CCR7+ M1 macrophages. This presents an attractive strategy for improving the design and fabrication of next-generation vaginal implants for pelvic reconstructive surgery.
Keywords: 3D‐printing; gynaecology; implant immune response; melt electrowriting; tissue engineering.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
