A VAMS-based LC-MS/MS method for precise cenobamate quantification in epilepsy (patients)
- PMID: 39297399
- PMCID: PMC11633685
- DOI: 10.1002/epi4.12927
A VAMS-based LC-MS/MS method for precise cenobamate quantification in epilepsy (patients)
Abstract
Objective: Cenobamate (CNB), a recently approved antiseizure medication by the European Medicines Agency (EMA), serves as an adjunctive therapy for focal-onset seizures in adult patients unresponsive to at least two other treatments. Administered in polytherapy, CNB can potentially interact with co-administered drugs in epilepsy patients, necessitating dose adjustments and the need for effective therapeutic drug monitoring (TDM).
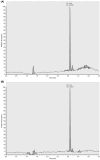
Methods: In this study, we introduce a novel LC-MS/MS method for precise CNB quantification using Volumetric Absorptive Microsampling (VAMS), following validation according to ICH guidelines M10. VAMS samples are efficiently extracted with 200 μL of methanol, with chromatographic separation achieved using an Acquity UPLC HSS PFP column. The method's efficacy was confirmed through its application to real samples from adult CNB-treated patients.
Results: Our results demonstrate that the method exhibits linearity within the range of 0.05-30 mg/L, with intra- and inter-run precision ranging from 1% to 8% and accuracy from 1% to 10% based on 30 μL of sample. Furthermore, CNB stability in VAMS is confirmed for up to 15 days at 25°C and -20°C. Importantly, no significant difference was observed between CNB concentrations in VAMS samples and those in plasma obtained from venous blood.
Significance: This VAMS-based LC-MS/MS method presents a robust alternative for TDM in CNB-treated patients. Future investigations should explore CNB concentrations in capillary blood and assess their correlation with plasma levels to further enhance its clinical utility.
Plain language summary: Cenobamate is an antiepileptic drug and used for treatment of focal-onset seizures in adult patients (≥18 age). TDM can prevent drug interactions and minimize drug toxicity. The aim of this work is to evaluate volumetric absorptive microsampling (VAMS) from capillary blood as an alternative strategy for TDM in patients treated with the newly antiepileptic drug. Our method is suitable for TDM, and this study suggests that VAMS allows monitoring of cenobamate concentration and can offer valuable support for personalized therapy in refractory epilepsy.
Keywords: antiseizure medications; cenobamate; drug monitoring; liquid chromatography tandem‐mass spectrometry; volumetric absorptive microsampling.
© 2024 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


References
-
- Ontozry | European Medicines Agency . 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ontozry. Accessed 5 Nov 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

