Ketamine Prevents Inflammation-Induced Reduction of Human Hippocampal Neurogenesis via Inhibiting the Production of Neurotoxic Metabolites of the Kynurenine Pathway
- PMID: 39297528
- PMCID: PMC11450635
- DOI: 10.1093/ijnp/pyae041
Ketamine Prevents Inflammation-Induced Reduction of Human Hippocampal Neurogenesis via Inhibiting the Production of Neurotoxic Metabolites of the Kynurenine Pathway
Abstract
Background: Understanding the precise mechanisms of ketamine is crucial for replicating its rapid antidepressant effects without inducing psychomimetic changes. Here, we explore whether the antidepressant-like effects of ketamine enantiomers are underscored by protection against cytokine-induced reductions in hippocampal neurogenesis and activation of the neurotoxic kynurenine pathway in our well-established in vitro model of depression in a dish.
Methods: We used the fetal hippocampal progenitor cell line (HPC0A07/03C) to investigate ketamine's impact on cytokine-induced reductions in neurogenesis in vitro. Cells were treated with interleukin- 1beta (IL-1b) (10 ng/mL) or IL-6 (50 pg/mL), alone or in combination with ketamine enantiomers arketamine (R-ketamine, 400 nM) or esketamine (S-ketamine, 400 nM) or antidepressants sertraline (1 mM) or venlafaxine (1 mM).
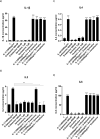
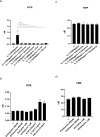
Results: Resembling the effect of antidepressants, both ketamine enantiomers prevented IL-1b- and IL-6-induced reduction in neurogenesis and increase in apoptosis. This was mediated by inhibition of IL-1b-induced production of IL-2 and IL-13 by R-ketamine and of IL-1b-induced tumor necrosis factor-alpha by S-ketamine. Likewise, R-ketamine inhibited IL-6-induced production of IL-13, whereas S-ketamine inhibited IL-6-induced IL-1b and IL-8. Moreover, both R- and S-ketamine prevented IL-1b-induced increases in indoleamine 2,3-dioxygenase expression as well as kynurenine production, which in turn was shown to mediate the detrimental effects of IL-1b on neurogenesis and apoptosis. In contrast, neither R- nor S-ketamine prevented IL-6-induced kynurenine pathway activation.
Conclusions: Results suggest that R- and S-ketamine have pro-neurogenic and anti-inflammatory properties; however, this is mediated by inhibition of the kynurenine pathway only in the context of IL-1b. Overall, this study enhances our understanding of the mechanisms underlying ketamine's antidepressant effects in the context of different inflammatory phenotypes, ultimately leading to the development of more effective, personalized therapeutic approaches for patients suffering from depression.
Keywords: Cytokines; apoptosis; hippocampal neurogenesis; kynurenine pathway.
© The Author(s) 2024. Published by Oxford University Press on behalf of CINP.
Figures


















References
-
- Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM (2013a) Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38:872–883. - PMC - PubMed
-
- Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, Thuret S, Price J, Uher R, Riva MA, Pariante CM (2013b) Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci USA 110:8708–8713. - PMC - PubMed
-
- Beilin B, Rusabrov Y, Shapira Y, Roytblat L, Greemberg L, Yardeni IZ, Bessler H (2007) Low-dose ketamine affects immune responses in humans during the early postoperative period. Br J Anaesth 99:522–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

