Characterization of galactose catabolic pathways in Streptococcus agalactiae and identification of a major galactose: phosphotransferase importer
- PMID: 39297619
- PMCID: PMC11500514
- DOI: 10.1128/jb.00155-24
Characterization of galactose catabolic pathways in Streptococcus agalactiae and identification of a major galactose: phosphotransferase importer
Abstract
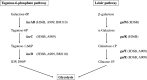
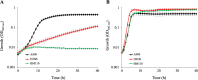
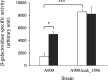
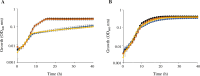
We identified and characterized genomic regions of Streptococcus agalactiae that are involved in the Leloir and the tagatose-6-phosphate pathways for D-galactose catabolism. The accumulation of mutations in genes coding the Leloir pathway and the absence of these genes in a significant proportion of the strains suggest that this pathway may no longer be necessary for S. agalactiae and is heading toward extinction. In contrast, a genomic region containing genes coding for intermediates of the tagatose-6-phosphate pathway, a Gat family PTS transporter, and a DeoR/GlpR family regulator is present in the vast majority of strains. By deleting genes that code for intermediates of each of these two pathways in three selected strains, we demonstrated that the tagatose-6-phosphate pathway is their sole route for galactose catabolism. Furthermore, we showed that the Gat family PTS transporter acts as the primary importer of galactose in S. agalactiae. Finally, we proved that the DeoR/GlpR family regulator is a repressor of the tagatose-6-phosphate pathway and that galactose triggers the induction of this biochemical mechanism.IMPORTANCES. agalactiae, a significant pathogen for both humans and animals, encounters galactose and galactosylated components within its various ecological niches. We highlighted the capability of this bacterium to metabolize D-galactose and showed the role of the tagatose-6-phosphate pathway and of a PTS importer in this biochemical process. Since S. agalactiae relies on carbohydrate fermentation for energy production, its ability to uptake and metabolize D-galactose could enhance its persistence and its competitiveness within the microbiome.
Keywords: Leloir pathway; PTS Gat family; environmental adaptation; galactose catabolism; group B streptococcus; phosphotransferase system; sugar metabolism; tagatose-6-phosphate pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Waldron KW, Faulds CB. 2007. Cell wall polysaccharides: composition and structure, p 181–201. In Kamerlin H (ed), Comprehensive glycoscience. Elsevier.
-
- Berry GT. 2000. Classic galactosemia and clinical variant galactosemia. In Adam MP, Mirzaa GM, Pagon RA (ed), GeneReviews. Vol. Available from. University of Washington, Seattle; 1993- 2023, Seattle (WA). https://www.ncbi.nlm.nih.gov/books/NBK1518/. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

