A cynomolgus monkey E. coli urinary tract infection model confirms efficacy of new FimH vaccine candidates
- PMID: 39297649
- PMCID: PMC11475676
- DOI: 10.1128/iai.00169-24
A cynomolgus monkey E. coli urinary tract infection model confirms efficacy of new FimH vaccine candidates
Abstract
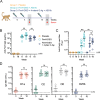
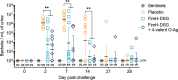
The increase in urinary tract infections (UTI) caused by antibiotic-resistant Escherichia coli requires the development of new therapeutic agents and prophylactic vaccines. To evaluate the efficacy of new lead candidates, we implemented a cynomolgus macaque UTI challenge model that mimics human uncomplicated cystitis in response to transurethral challenge with a multidrug-resistant (MDR) E. coli serotype O25b ST131 isolate. E. coli fimbrial adhesin FimH and O-antigens are separately under clinical evaluation by others as vaccine candidates to prevent UTI and invasive urosepsis disease, respectively. Accordingly, we assessed the protective efficacy of three 50-µg intramuscular doses of a novel recombinant FimH antigen adjuvanted with liposomal QS21/MPLA compared with saline placebo in groups of nine animals. A third group was vaccinated with this FimH formulation in combination with 1 µg each of a four-valent mixture of serotype O1a, O2, O6, and O25b O-antigen CRM197 lattice glycoconjugates. Both vaccines elicited high levels of serum FimH IgG and adhesin blocking antibodies at the time of bacterial challenge and, for the combination group, O-antigen-specific antibodies. Following bacterial challenge, both vaccinated groups showed >200- and >700-fold reduction in bacteriuria at day 2 and day 7 post-infection compared with placebo, respectively. In parallel, both vaccines significantly reduced levels of inflammatory biomarkers IL-8 and myeloperoxidase in the urine at day 2 post-infection relative to placebo. Results provide preclinical proof-of-concept for the prevention of an MDR UTI infection by these new vaccine formulations.
Keywords: Escherichia coli; FimH; UPEC; UTI; cystitis; non-human primate.
Conflict of interest statement
This study was supported by Pfizer Inc. All authors were employees of Pfizer Inc. during this study and some authors are Pfizer stock owners. Pfizer was involved in the design, analysis, and interpretation of the data in this research study, the writing of this report, and the decision to publish. R.G.K.D., N.C.S.D.M., L.C., and A.S.A. are inventors on related patents.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

