Immunity to Non-Dengue Flaviviruses Impacts Dengue Virus Immunoglobulin G Enzyme-Linked Immunosorbent Assay Specificity in Cambodia
- PMID: 39297691
- PMCID: PMC11841641
- DOI: 10.1093/infdis/jiae422
Immunity to Non-Dengue Flaviviruses Impacts Dengue Virus Immunoglobulin G Enzyme-Linked Immunosorbent Assay Specificity in Cambodia
Erratum in
-
Correction to: Immunity to Non-Dengue Flaviviruses Impacts Dengue Virus Immunoglobulin G Enzyme-Linked Immunosorbent Assay Specificity in Cambodia.J Infect Dis. 2024 Dec 16;230(6):e1415. doi: 10.1093/infdis/jiae492. J Infect Dis. 2024. PMID: 39405128 Free PMC article. No abstract available.
Abstract
Background: Seroprevalence studies are the standard for disease surveillance, and serology determined eligibility for the first dengue vaccine. Expanding flavivirus co-circulation and vaccination complicate testing. We evaluate the accuracy of a common dengue virus serological assay, examine immunity to non-dengue flaviviruses as a contributor to decreased performance, and assess whether alternative cut points may improve assay performance.
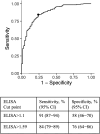
Methods: Children (n = 770) aged 2-9 years in Kampong Speu, Cambodia were enrolled in a prospective longitudinal study, and PanBio indirect dengue virus immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) was performed. Plaque reduction neutralization tests (PRNTs) using dengue viruses were performed on a subset to assess the accuracy of the IgG ELISA, and PRNTs with Zika, Japanese encephalitis, and West Nile viruses evaluated immunity to non-dengue flaviviruses. Receiver operating curve analysis identified an alternative cut point to improve IgG ELISA accuracy.
Results: The dengue IgG ELISA had a lower specificity than previously reported (58% vs 93%-100%). Of those with false-positive IgG results, 46% had detectable neutralizing antibodies against other flaviviruses including 14% against West Nile virus. A higher IgG cut point improved the test accuracy in this population.
Conclusions: Physicians and public health authorities should be alert for West Nile in Cambodia. Immunity to non-dengue flaviviruses can impact dengue surveillance.
Clinical trials registration: NCT03534245.
Keywords: West Nile virus; cross-reactivity; dengue virus; seroprevalence; specificity.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. E. M. is now a Clinical Franchise Leader of Influenza in Vaccines Research and Development at Sanofi Pasteur. The work reported in this manuscript was conducted prior to her switching positions. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Update of
-
Immunity to non-dengue flaviviruses impacts dengue virus IgG ELISA specificity in Cambodia.medRxiv [Preprint]. 2023 Nov 20:2023.11.17.23298701. doi: 10.1101/2023.11.17.23298701. medRxiv. 2023. Update in: J Infect Dis. 2025 Feb 20;231(2):e337-e344. doi: 10.1093/infdis/jiae422. PMID: 38076831 Free PMC article. Updated. Preprint.
References
-
- Postler TS, Beer M, Blitvich BJ, et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch Virol 2023; 168:224. - PubMed
-
- Strategic Advisory Group of Experts on Immunization . Background paper on dengue vaccines. https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Apr2016/10_.... Accessed 17 November 2023.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

