Interleukin-33-activated basophils promote asthma by regulating Th2 cell entry into lung tissue
- PMID: 39297875
- PMCID: PMC11413418
- DOI: 10.1084/jem.20240103
Interleukin-33-activated basophils promote asthma by regulating Th2 cell entry into lung tissue
Abstract
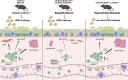
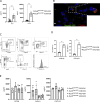
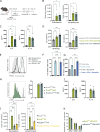
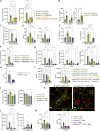
Asthma is characterized by lung eosinophilia, remodeling, and mucus plugging, controlled by adaptive Th2 effector cells secreting IL-4, IL-5, and IL-13. Inhaled house dust mite (HDM) causes the release of barrier epithelial cytokines that activate various innate immune cells like DCs and basophils that can promote Th2 adaptive immunity directly or indirectly. Here, we show that basophils play a crucial role in the development of type 2 immunity and eosinophilic inflammation, mucus production, and bronchial hyperreactivity in response to HDM inhalation in C57Bl/6 mice. Interestingly, conditional depletion of basophils during sensitization did not reduce Th2 priming or asthma inception, whereas depletion during allergen challenge did. During the challenge of sensitized mice, basophil-intrinsic IL-33/ST2 signaling, and not FcεRI engagement, promoted basophil IL-4 production and subsequent Th2 cell recruitment to the lungs via vascular integrin expression. Basophil-intrinsic loss of the ubiquitin modifying molecule Tnfaip3, involved in dampening IL-33 signaling, enhanced key asthma features. Thus, IL-33-activated basophils are gatekeepers that boost allergic airway inflammation by controlling Th2 tissue entry.
© 2024 Schuijs et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





Similar articles
-
The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma.Clin Exp Allergy. 2016 Mar;46(3):479-90. doi: 10.1111/cea.12683. Clin Exp Allergy. 2016. PMID: 26609909
-
Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen.J Exp Med. 2010 Sep 27;207(10):2097-111. doi: 10.1084/jem.20101563. Epub 2010 Sep 6. J Exp Med. 2010. PMID: 20819925 Free PMC article.
-
TNF-α-induced protein 3 levels in lung dendritic cells instruct TH2 or TH17 cell differentiation in eosinophilic or neutrophilic asthma.J Allergy Clin Immunol. 2018 May;141(5):1620-1633.e12. doi: 10.1016/j.jaci.2017.08.012. Epub 2017 Sep 6. J Allergy Clin Immunol. 2018. PMID: 28888782
-
Innate and adaptive type 2 immunity in lung allergic inflammation.Immunol Rev. 2017 Jul;278(1):162-172. doi: 10.1111/imr.12557. Immunol Rev. 2017. PMID: 28658559 Review.
-
Basophils and type 2 immunity.Curr Opin Hematol. 2008 Jan;15(1):59-63. doi: 10.1097/MOH.0b013e3282f13ce8. Curr Opin Hematol. 2008. PMID: 18043247 Free PMC article. Review.
Cited by
-
Targeting γc family cytokines with biologics: current status and future prospects.MAbs. 2025 Dec;17(1):2468312. doi: 10.1080/19420862.2025.2468312. Epub 2025 Feb 18. MAbs. 2025. PMID: 39967341 Free PMC article. Review.
-
Regulation of eosinophil recruitment and heterogeneity during allergic airway inflammation.Front Allergy. 2025 Apr 10;6:1585142. doi: 10.3389/falgy.2025.1585142. eCollection 2025. Front Allergy. 2025. PMID: 40276331 Free PMC article. Review.
-
The immunology of asthma.Nat Immunol. 2025 Aug;26(8):1233-1245. doi: 10.1038/s41590-025-02212-9. Epub 2025 Jul 29. Nat Immunol. 2025. PMID: 40730897 Review.
-
Epithelial-immune interactions govern type 2 immunity at barrier surfaces.Curr Opin Immunol. 2024 Dec;91:102501. doi: 10.1016/j.coi.2024.102501. Epub 2024 Nov 10. Curr Opin Immunol. 2024. PMID: 39522453 Review.
-
The Importance of Lung Innate Immunity During Health and Disease.Pathogens. 2025 Jan 17;14(1):91. doi: 10.3390/pathogens14010091. Pathogens. 2025. PMID: 39861052 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

