Landiolol for heart rate control in patients with septic shock and persistent tachycardia. A multicenter randomized clinical trial (Landi-SEP)
- PMID: 39297945
- PMCID: PMC11447033
- DOI: 10.1007/s00134-024-07587-1
Landiolol for heart rate control in patients with septic shock and persistent tachycardia. A multicenter randomized clinical trial (Landi-SEP)
Abstract
Purpose: Excessive tachycardia in resuscitated septic shock patients can impair hemodynamics and worsen patient outcome. We investigated whether heart rate (HR) control can be achieved without increased vasopressor requirements using the titratable highly selective, ultra-short-acting β1-blocker landiolol.
Methods: This randomized, open-label, controlled trial was conducted at 20 sites in 7 European countries from 2018 to 2022 and investigated the efficacy and safety of landiolol in adult patients with septic shock and persistent tachycardia. Patients were randomly assigned to receive either landiolol along with standard treatment (n = 99) or standard treatment alone (n = 101). The combined primary endpoint was HR response (i.e., HR within the range of 80-94 beats per minute) and its maintenance without increasing vasopressor requirements during the first 24 h after treatment start. Key secondary endpoints were 28-day mortality and adverse events.
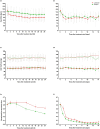
Results: Out of 196 included septic shock patients, 98 received standard treatment combined with landiolol and 98 standard treatment alone. A significantly larger proportion of patients met the combined primary endpoint in the landiolol group than in the control group (39.8% [39/98] vs. 23.5% [23/98]), with a between-group difference of 16.5% (95% confidence interval [CI]: 3.4-28.8%; p = 0.013). There were no statistically significant differences between study groups in tested secondary outcomes and adverse events.
Conclusion: The ultra-short-acting beta-blocker landiolol was effective in reducing and maintaining HR without increasing vasopressor requirements after 24 h in patients with septic shock and persistent tachycardia. There were no differences in adverse events and clinical outcomes such as 28-day mortality vs. standard of care. The results of this study, in the context of previous trials, do not support a treatment strategy of stringent HR reduction (< 95 bpm) in an unselected septic shock population with persistent tachycardia. Further investigations are needed to identify septic shock patient phenotypes that benefit clinically from HR control.
Keywords: Heart rate control; Landiolol; Persistent tachycardia; Sepsis; Septic shock; Ultra-short-acting beta-blocker.
© 2024. The Author(s).
Conflict of interest statement
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. MU, JS, CK, KK, NKB are employees of AOP Orphan Pharmaceuticals GmbH. GK is a Board Director of AOP Health International Management AG.
Figures
References
-
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Moller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47:1181–1247 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

