Reactions of citrinin with amino compounds modelling thermal food processing
- PMID: 39298071
- PMCID: PMC11480111
- DOI: 10.1007/s12550-024-00557-y
Reactions of citrinin with amino compounds modelling thermal food processing
Abstract
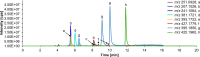
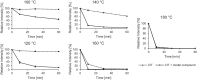
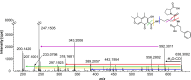
Citrinin (CIT) is a nephrotoxic mycotoxin, produced by several species of Penicillium, Aspergillus, and Monascus. The foodstuffs most frequently contaminated with CIT include cereals, cereal products, and red yeast rice. Studies on the occurrence of CIT in food have shown that the CIT concentrations in processed cereal-based products are generally lower than in unprocessed industry cereal samples. One possible explanation is the reaction of CIT with major food components such as carbohydrates or proteins to form modified CIT. Such modified forms of CIT are then hidden from conventional analyses, but it is possible that they are converted back into the parent mycotoxin during digestion. The aim of this study is therefore to investigate reactions of CIT with food matrix during thermal processes and to gain a deeper understanding of the degradation of CIT during food processing. In this study, we could demonstrate that CIT reacts with amino compounds such as proteins, under typical food processing conditions, leading to modified forms of CIT.
Keywords: Biomonitoring; Citrinin; Degradation; Food; Mycotoxin; Stability.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
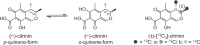
References
-
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

