Stereotactic vs Hypofractionated Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: The LUSTRE Phase 3 Randomized Clinical Trial
- PMID: 39298144
- PMCID: PMC11413752
- DOI: 10.1001/jamaoncol.2024.3089
Stereotactic vs Hypofractionated Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: The LUSTRE Phase 3 Randomized Clinical Trial
Abstract
Importance: Stereotactic body radiotherapy (SBRT) is widely used for stage I medically inoperable non-small cell lung cancer (NSCLC), yet varied results from randomized clinical trials (RCTs) and concerns in treating centrally located tumors persist.
Objective: To examine whether SBRT would improve local control (LC) compared with hypofractionated conventional radiotherapy (CRT).
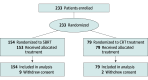
Design setting and participants: This phase 3 RCT was conducted in 16 Canadian centers. Patients with medically inoperable stage I (≤5 cm) NSCLC were randomized 2:1 to SBRT of 48 Gy in 4 fractions (peripheral NSCLC) or 60 Gy in 8 fractions (central NSCLC) vs CRT of 60 Gy in 15 fractions. Data were collected from May 2014 to January 2020, and data were analyzed from July 2022 to July 2023.
Interventions: SBRT or CRT.
Main outcomes and measures: The primary objective was to determine the effectiveness of SBRT compared with CRT based on LC at 3 years. Secondary outcomes included event-free survival, overall survival, and toxic effects. All radiation plans were subject to real-time/final review. Local failures were centrally adjudicated. The study was designed to detect a 3-year LC improvement of SBRT from 75% to 87.5%. The target sample size was 324 patients.
Results: Of 233 included patients, 119 (51.1%) were male, and the mean (SD) age was 75.4 (7.7) years; the median (IQR) follow-up was 36.1 (26.4-52.8) months. A total of 154 patients received SBRT and 79 received CRT. The 3-year LC was 87.6% (95% CI, 81.9%-93.4%) for SBRT and 81.2% (95% CI, 71.9%-90.5%) for CRT (hazard ratio [HR], 0.61; 95% CI, 0.31-1.20; P = .15). The HR was 1.02 (95% CI, 0.72-1.45; P = .87) for event-free survival and 1.18 (95% CI, 0.80-1.76; P = .40) for overall survival. Minimal acute toxic effects were observed. Among those randomized to SBRT, late grade 3 or 4 toxic effects occurred in 5 of 45 (11%) with central NSCLC and 2 of 109 (1.8%) with peripheral NSCLC; among those randomized to CRT, in 1 of 19 (5%) with central NSCLC and 1 of 60 (2%) with peripheral NSCLC. One patient who received SBRT for an ultracentral lesion (target overlapping proximal bronchus) experienced a possible treatment-related grade 5 event (hemoptysis).
Conclusions and relevance: This RCT compared lung SBRT with hypofractionated CRT that included central/ultracentral tumors. No difference was detected in LC between groups. Severe toxic effects were limited, including patients with central tumors. The trial provides important prospective data evaluating SBRT; however, further research is necessary to determine if SBRT is more effective than CRT for peripheral and central NSCLC.
Trial registration: ClinicalTrials.gov Identifier: NCT03924869.
Copyright 2024 Swaminath A et al. JAMA Oncology.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Swaminath reported personal fees from AstraZeneca, Bristol Myers Squibb, and Eisai outside the submitted work. Dr Bujold reported grants from Ontario Clinical Oncology Group during the conduct of the study. Dr Gabos reported personal fees from AstraZeneca outside the submitted work. Dr Mehiri reported grants from Ontario Clinical Oncology Group during the conduct of the study. Dr Louie reported personal fees from AstraZeneca outside the submitted work. Dr Whelan reported nonfinancial support from Exact Science outside the submitted work. No other disclosures were reported.
Figures
Comment in
- doi: 10.1001/jamaoncol.2024.2905
References
-
- Ball D, Mai GT, Vinod S, et al. ; TROG 09.02 CHISEL investigators . Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494-503. doi:10.1016/S1470-2045(18)30896-9 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials