Red Blood Cell Transfusion in European Neonatal Intensive Care Units, 2022 to 2023
- PMID: 39298172
- PMCID: PMC11413711
- DOI: 10.1001/jamanetworkopen.2024.34077
Red Blood Cell Transfusion in European Neonatal Intensive Care Units, 2022 to 2023
Erratum in
-
Error in Supplement 1 and Supplement 2.JAMA Netw Open. 2024 Oct 1;7(10):e2445861. doi: 10.1001/jamanetworkopen.2024.45861. JAMA Netw Open. 2024. PMID: 39441602 Free PMC article. No abstract available.
Abstract
Importance: Red blood cell (RBC) transfusions are frequently administered to preterm infants born before 32 weeks of gestation in the neonatal intensive care unit (NICU). Two randomized clinical trials (Effects of Transfusion Thresholds on Neurocognitive Outcomes of Extremely Low-Birth-Weight Infants [ETTNO] and Transfusion of Prematures [TOP]) found that liberal RBC transfusion thresholds are nonsuperior to restrictive thresholds, but the extent to which these results have been integrated into clinical practice since publication in 2020 is unknown.
Objective: To describe neonatal RBC transfusion practice in Europe.
Design, setting, and participants: This international prospective observational cohort study collected data between September 1, 2022, and August 31, 2023, with a 6-week observation period per center, from 64 NICUs in 22 European countries. Participants included 1143 preterm infants born before 32 weeks of gestation.
Exposure: Admission to the NICU.
Main outcomes and measures: Study outcome measures included RBC transfusion prevalence rates, cumulative incidence, indications, pretransfusion hemoglobin (Hb) levels, volumes, and transfusion rates, Hb increment, and adverse effects of RBC transfusion.
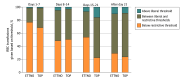
Results: A total of 1143 preterm infants were included (641 male [56.1%]; median gestational age at birth, 28 weeks plus 2 days [IQR, 26 weeks plus 2 days to 30 weeks plus 2 days]; median birth weight, 1030 [IQR, 780-1350] g), of whom 396 received 1 or more RBC transfusions, totaling 903 transfusions. Overall RBC transfusion prevalence rate during postnatal days 1 to 28 was 3.4 transfusion days per 100 admission days, with considerable variation across countries, only partly explained by patient mix. By day 28, 36.5% (95% CI, 31.6%-41.5%) of infants had received at least 1 transfusion. Most transfusions were given based on a defined Hb threshold (748 [82.8%]). Hemoglobin levels before transfusions indicated for threshold were below the restrictive thresholds set by ETTNO in 324 of 729 transfusions (44.4%) and TOP in 265 of 729 (36.4%). Conversely, they were between restrictive and liberal thresholds in 352 (48.3%) and 409 (56.1%) transfusions, respectively, and above liberal thresholds in 53 (7.3%) and 55 (7.5%) transfusions, respectively. Most transfusions given based on threshold had volumes of 15 mL/kg (470 of 738 [63.7%]) and were administered over 3 hours (400 of 738 [54.2%]), but there was substantial variation in dose and duration.
Conclusions and relevance: In this cohort study of very preterm infants, most transfusions indicated for threshold were given for pretransfusion Hb levels above restrictive transfusion thresholds evaluated in recent trials. These results underline the need to optimize practices and for implementation research to support uptake of evidence.
Conflict of interest statement
Figures



References
-
- Franz AR, Engel C, Bassler D, et al. ; ETTNO Investigators . Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA. 2020;324(6):560-570. doi:10.1001/jama.2020.10690 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

